



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
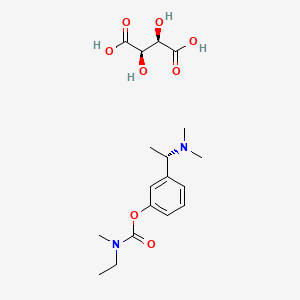

1. (s)-n-ethyl-3-((1-dimethyl-amino)ethyl)-n-methylphenylcarbamate
2. 713, Ena
3. 713, Sdz Ena
4. Ena 713
5. Ena 713, Sdz
6. Ena-713
7. Ena713
8. Exelon
9. Hydrogen Tartrate, Rivastigmine
10. Rivastigmine
11. Rivastigmine Hydrogen Tartrate
12. Rivastigminetartrate
13. Sdz Ena 713
14. Tartrate, Rivastigmine Hydrogen
1. 129101-54-8
2. Rivastigmine Hydrogen Tartrate
3. Ena 713
4. Sdz-ena 713
5. Rivastigmine Bitartrate
6. Rivastigmine (tartrate)
7. (s)-3-(1-(dimethylamino)ethyl)phenyl Ethyl(methyl)carbamate (2r,3r)-2,3-dihydroxysuccinate
8. Rivastigmine Hydrogentartrate
9. Rivastigmine Tartrate (exelon)
10. 9iy2357jpe
11. Chebi:64358
12. (2r,3r)-2,3-dihydroxybutanedioic Acid;[3-[(1s)-1-(dimethylamino)ethyl]phenyl] N-ethyl-n-methylcarbamate
13. Rivastigmine Actavis
14. (2r,3r)-2,3-dihydroxybutanedioic Acid 3-[(1s)-1-(dimethylamino)ethyl]phenyl N-ethyl-n-methylcarbamate
15. Carbamic Acid, N-ethyl-n-methyl-, 3-[(1s)-1-(dimethylamino)ethyl]phenyl Ester, (2r,3r)-2,3-dihydroxybutanedioate (1:1)
16. Rivastigmine Tartrate Salt
17. Unii-9iy2357jpe
18. Rivastach
19. Exelon Mr
20. Exelon Tds
21. Nimvastid (tn)
22. Carbamic Acid, N-ethyl-n-methyl-, 3-((1s)-1-(dimethylamino)ethyl)phenyl Ester, (2r,3r)-2,3-dihydroxybutanedioate (1:1)
23. Rivastigmine Tartrate;
24. Rivastigmine L-tartrate
25. Rivastigmine Tartrate [usp]
26. Rivastigmine Tartrate- Bio-x
27. Mls006011143
28. Chembl1201092
29. Hms3678f17
30. Hms3884h05
31. Rivastigmine For System Suitability
32. Act03355
33. Mfcd03700731
34. Rivastigmine Tartrate [vandf]
35. S2087
36. Akos022179413
37. Rivastigmine Tartrate [usp-rs]
38. Ac-3487
39. Ccg-213410
40. Cs-0947
41. T01r548
42. As-13921
43. Br164357
44. Carbamic Acid, Ethylmethyl-, 3-((1s)-1-(dimethylamino)ethyl)phenyl Ester, (2r,3r)-2,3-dihydroxybutanedioate (1:1)
45. Carbamic Acid, Ethylmethyl-, 3-(1-(dimethylamino)ethyl)phenyl Ester, (s)-, (r-(r*,r*))-2,3-dihydroxybutanedioate (1:1)
46. Hy-11017
47. N-ethyl-n-methylcarbamic Acid 3-[(1s)-1-(dimethylamino)ethyl]phenyl Ester (2r,3r)-2,3-dihydroxybutanedioate
48. Smr004702920
49. Rivastigmine (as Hydrogen Tartrate)
50. Rivastigmine Hydrogen Tartrate [mi]
51. Rivastigmine Tartrate [orange Book]
52. Am20080949
53. R0093
54. Rivastigmine Tartrate [usp Monograph]
55. Sw199663-2
56. Rivastigmine Hydrogen Tartrate [mart.]
57. D02558
58. Rivastigmine Hydrogen Tartrate [who-dd]
59. Rivastigmine Hydrogen Tartrate [ema Epar]
60. Q-101313
61. Rivastigmine Hydrogen Tartrate [ep Monograph]
62. Q27133236
63. 3-[(s)-1-(dimethylamino)ethyl]phenyl N-ethyl-n-methylcarbamate L-tartrate
64. N-ethyl-n-methylcarbamic Acid 3-[(s)-1-(dimethylamino)ethyl]phenyl Ester L-tartrate
65. (1s)-1-(3-{[ethyl(methyl)carbamoyl]oxy}phenyl)-n,n-dimethylethanaminium (2r,3r)-3-carboxy-2,3-dihydroxypropanoate
66. 3-[(1s)-1-(dimethylamino)ethyl]phenyl Ethyl(methyl)carbamate (2r,3r)-2,3-dihydroxybutanedioate
| Molecular Weight | 400.4 g/mol |
|---|---|
| Molecular Formula | C18H28N2O8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 400.18456586 g/mol |
| Monoisotopic Mass | 400.18456586 g/mol |
| Topological Polar Surface Area | 148 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 402 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Rivastigmine tartrate |
| Drug Label | Rivastigmine tartrate is a reversible cholinesterase inhibitor and is known chemically as (S)-N-Ethyl-N-methyl-3-[1-(dimethylamino)ethyl]-phenyl carbamate hydrogen-(2R,3R)-tartrate. Rivastigmine tartrate is commonly referred to in the pharmacological... |
| Active Ingredient | Rivastigmine tartrate |
| Dosage Form | Capsule |
| Route | oral; Oral |
| Strength | eq 4.5mg base; eq 6mg base; eq 1.5mg base; eq 3mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Apotex; Alembic Pharms; Sun Pharm Inds; Watson Labs; Macleods Pharms; Dr Reddys Labs; Orchid Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Rivastigmine tartrate |
| Drug Label | Rivastigmine tartrate is a reversible cholinesterase inhibitor and is known chemically as (S)-N-Ethyl-N-methyl-3-[1-(dimethylamino)ethyl]-phenyl carbamate hydrogen-(2R,3R)-tartrate. Rivastigmine tartrate is commonly referred to in the pharmacological... |
| Active Ingredient | Rivastigmine tartrate |
| Dosage Form | Capsule |
| Route | oral; Oral |
| Strength | eq 4.5mg base; eq 6mg base; eq 1.5mg base; eq 3mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Apotex; Alembic Pharms; Sun Pharm Inds; Watson Labs; Macleods Pharms; Dr Reddys Labs; Orchid Hlthcare |
Symptomatic treatment of mild to moderately severe Alzheimer's dementia.
Symptomatic treatment of mild to moderately severe dementia in patients with idiopathic Parkinson's disease.
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
N06DA03








