



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
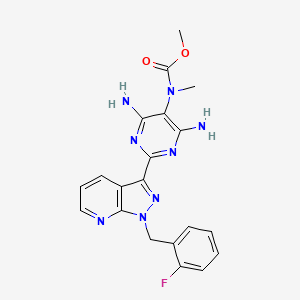

1. Adempas
2. Bay 63-2521
3. Bay-63-2521
1. 625115-55-1
2. Adempas
3. Bay 63-2521
4. Riociguat (bay 63-2521)
5. Bay-63-2521
6. Ru3fe2y4xi
7. Methyl (4,6-diamino-2-(1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)(methyl)carbamate
8. Methyl N-[4,6-diamino-2-[1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]-n-methylcarbamate
9. Chebi:76018
10. 1304478-72-5
11. N-[4,6-diamino-2-[1-[(2-fluorophenyl)methyl]-1h-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-n-methylcarbamic Acid Methyl Ester
12. Methyl N-(4,6-diamino-2-{1-((2-fluorophenyl)methyl)-1h-pyrazolo(3,4-b)pyridin-3-yl}pyrimidin-5-yl)-n-methylcarbamate
13. Methyl N-(4,6-diamino-2-{1-[(2-fluorophenyl)methyl]-1h-pyrazolo[3,4-b]pyridin-3-yl}pyrimidin-5-yl)-n-methylcarbamate
14. Riociguat [inn]
15. Methyl ~{n}-[4,6-bis(azanyl)-2-[1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]-~{n}-methyl-carbamate
16. Methyl 4,6-diamino-2-(1-(2-fluorobenzyl)-1h-pyrazolo(3,4-b)pyridin-3-yl)-5-pyrimidinyl(methyl)carbamate
17. Unii-ru3fe2y4xi
18. Riociguatum
19. Riociguate
20. Riociguat [usan:inn:jan]
21. Riocguat-d3
22. Methyl 4,6-diamino-2-[1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl(methyl)carbamate
23. Methyl N-(4,6-diamino-2-(1-((2-fluorophenyl)methyl)-1h-pyrazolo(3,4-b)pyridin-3-yl)pyrimidin-5-yl)-n-methylcarbamate
24. Adempas (tn)
25. Bay 632521
26. Riociguat [jan]
27. Riociguat [mi]
28. Riociguat [usan]
29. Riociguat [vandf]
30. Riociguat [usp-rs]
31. Riociguat [who-dd]
32. Bay 63-2521,riociguat
33. Riociguat (jan/usan/inn)
34. Schembl245457
35. Gtpl5257
36. Riociguat [orange Book]
37. Chembl2107834
38. Riociguat [ep Monograph]
39. Amy4218
40. Dtxsid50978109
41. Riociguat [usp Monograph]
42. Bcp04750
43. Bcp23980
44. Ex-a2023
45. Zinc3819392
46. Riociguat,cas:625115-55-1
47. Mfcd19443708
48. S8135
49. Akos015900718
50. Akos032950011
51. Bay-632521
52. Bcp9000382
53. Ccg-268912
54. Cs-0584
55. Db08931
56. Pb25734
57. Ncgc00379065-03
58. Ncgc00379065-04
59. Ncgc00379065-05
60. [4,6-diamino-2-[1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]methylcarbamic Acid Methyl Ester
61. Ac-27647
62. As-19299
63. Hy-14779
64. Bcp0726000023
65. Db-073136
66. Ft-0760451
67. Bay 63-2521;bay 632521
68. D09572
69. Bay 63-2521; Bay 632521
70. 115r551
71. Q2154494
72. Bay 63-2521-d3; Bay 632521-d3; Methyl [4,6-diamino-2-[1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]methylcarbamate-d3
73. Carbamic Acid, (4,6-diamino-2-(1-((2-fluorophenyl)methyl)-1h-pyrazolo(3,4-b)pyridin-3-yl)-5-pyrimidinyl)methyl-, Methyl Ester
74. Carbamic Acid, N-(4,6-diamino-2-(1-((2-fluorophenyl)methyl)-1h-pyrazolo(3,4-b)pyridin-3-yl)-5-pyrimidinyl)-n-methyl-, Methyl Ester
75. Gzo
76. Methyl {4,6-diamino-2-[1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}methylcarbamate
77. Methyl N-[4,6-diamino-2-[1-[(2-fluorophenyl)methyl]-1h-pyrazolo[3,4-b]pyridin-3-yl]-5-pyrimidinyl]-n-methyl-carbaminate
| Molecular Weight | 422.4 g/mol |
|---|---|
| Molecular Formula | C20H19FN8O2 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 5 |
| Exact Mass | 422.16150004 g/mol |
| Monoisotopic Mass | 422.16150004 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 618 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Adempas |
| PubMed Health | Riociguat (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Adempas (riociguat) is a tablet for oral administration. Riociguat is methyl 4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo [3,4-b]pyridin-3-yl]-5-pyrimidinyl(methyl)carbamate with the following structural formula:C20H19FN8O2Riociguat is a white to ye... |
| Active Ingredient | Riociguat |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 1.5mg; 0.5mg; 2mg; 1mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Adempas |
| PubMed Health | Riociguat (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Adempas (riociguat) is a tablet for oral administration. Riociguat is methyl 4,6-diamino-2-[1-(2-fluorobenzyl)-1H-pyrazolo [3,4-b]pyridin-3-yl]-5-pyrimidinyl(methyl)carbamate with the following structural formula:C20H19FN8O2Riociguat is a white to ye... |
| Active Ingredient | Riociguat |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 1.5mg; 0.5mg; 2mg; 1mg |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
Riociguat is indicated for the treatment of adults with persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH), (WHO Group 4) after surgical treatment, or inoperable CTEPH, to improve exercise capacity and WHO functional class. Riociguat is indicated for the treatment of adults with pulmonary arterial hypertension (PAH), (WHO Group 1), to improve exercise capacity, WHO functional class and to delay clinical worsening. Efficacy was shown in patients on Riociguat monotherapy or in combination with endothelin receptor antagonists or prostanoids. Studies establishing effectiveness included predominately patients with WHO functional class IIIII and etiologies of idiopathic or heritable PAH (61%) or PAH associated with connective tissue diseases (25%).
FDA Label
Chronic thromboembolic pulmonary hypertension (CTEPH)
Adempas is indicated for the treatment of adult patients with WHO Functional Class (FC) II to III with
- inoperable CTEPH,
- persistent or recurrent CTEPH after surgical treatment,
to improve exercise capacity (see section 5. 1).
Pulmonary arterial hypertension (PAH)
Adempas, as monotherapy or in combination with endothelin receptor antagonists, is indicated for the
treatment of adult patients with pulmonary arterial hypertension (PAH) with WHO Functional Class
(FC) II to III to improve exercise capacity.
Efficacy has been shown in a PAH population including aetiologies of idiopathic or heritable PAH or
PAH associated with connective tissue disease (see section 5. 1).
Enzyme Activators
Compounds or factors that act on a specific enzyme to increase its activity. (See all compounds classified as Enzyme Activators.)
C02KX05
C - Cardiovascular system
C02 - Antihypertensives
C02K - Other antihypertensives
C02KX - Antihypertensives for pulmonary arterial hypertension
C02KX05 - Riociguat
Absorption
The pharmacokinetics of riociguant are dose proportional from 0.5 mg to 2.5 mg. The absolute bioavailability is approximately 94%. After oral administration, peak plasma concentrations were achieved within 1.5 hours. Food does not affect the bioavailability of riociguat.
Route of Elimination
Riociguat is eliminated in the urine (40%) and feces (53%), largely as metabolites.
Volume of Distribution
Volume of distribution at steady state = 30 L
The active metabolite (M1) of riociguat is 1/3 to 1/10 as potent as riociguat.
About 12 hours in patients and 7 hours in healthy subjects.
Riociguat is a stimulator of soluble guanylate cyclase (sGC), an enzyme in the cardiopulmonary system and the receptor for nitric oxide (NO). When NO binds to sGC, the enzyme catalyzes synthesis of the signaling molecule cyclic guanosine monophosphate (cGMP). Intracellular cGMP plays an important role in regulating processes that influence vascular tone, proliferation, fibrosis and inflammation. Pulmonary hypertension is associated with endothelial dysfunction, impaired synthesis of nitric oxide and insufficient stimulation of the NO-sGC-cGMP pathway. Riociguat has a dual mode of action. It sensitizes sGC to endogenous NO by stabilizing the NO-sGC binding. Riociguat also directly stimulates sGC via a different binding site, independently of NO. Riociguat stimulates the NO-sGC-cGMP pathway and leads to increased generation of cGMP with subsequent vasodilation.






