



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
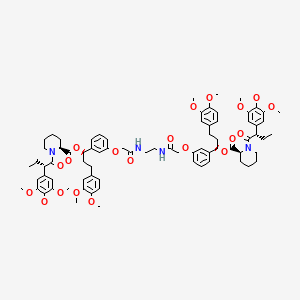

1. 2-piperidinecarboxylic Acid, 1-((2s)-1-oxo-2-(3,4,5-trimethoxyphenyl)butyl)-, 2,2'-(1,2-ethanediylbis(imino(2-oxo-2,1-ethanediyl)oxy-3,1-phenylene((1r)-3-(3,4-dimethoxyphenyl)propylidene))) Ester, (2s,2's)-
2. Ap 1903 Reagent
3. Ap1903
1. Ap1903
2. 195514-63-7
3. Ap-1903
4. Rimiducid [inn]
5. Ap 1903
6. H564l1w5j2
7. [(1r)-3-(3,4-dimethoxyphenyl)-1-[3-[2-[2-[[2-[3-[(1r)-3-(3,4-dimethoxyphenyl)-1-[(2s)-1-[(2s)-2-(3,4,5-trimethoxyphenyl)butanoyl]piperidine-2-carbonyl]oxypropyl]phenoxy]acetyl]amino]ethylamino]-2-oxoethoxy]phenyl]propyl] (2s)-1-[(2s)-2-(3,4,5-trimethoxyphenyl)butanoyl]piperidine-2-carboxylate
8. (1r,1'r)-((((ethane-1,2-diylbis(azanediyl))bis(2-oxoethane-2,1-diyl))bis(oxy))bis(3,1-phenylene))bis(3-(3,4-dimethoxyphenyl)propane-1,1-diyl) (2s,2's)-bis(1-((s)-2-(3,4,5-trimethoxyphenyl)butanoyl)piperidine-2-carboxylate)
9. 2-piperidinecarboxylic Acid, 1-((2s)-1-oxo-2-(3,4,5-trimethoxyphenyl)butyl)-, 2,2'-(1,2-ethanediylbis(imino(2-oxo-2,1-ethanediyl)oxy-3,1-phenylene((1r)-3-(3,4-dimethoxyphenyl)propylidene))) Ester, (2s,2's)-
10. Unii-h564l1w5j2
11. Ap 1903 Reagent
12. Rimiducid (usan/inn)
13. Rimiducid [usan:inn]
14. Rimiducid [usan]
15. Rimiducid (ap1903)
16. Ap 1903new
17. Rimiducid [who-dd]
18. Chembl269259
19. Schembl10111062
20. Dtxsid80173226
21. Ex-a1711
22. Vha51463
23. Ap1903ap1903
24. S9726
25. Cs-3162
26. Db04974
27. Ac-35487
28. Hy-16046
29. D11195
30. A919658
31. Q27279658
32. (1r,1'r)-((((ethane-1,2-diylbis(azanediyl))bis(2-oxoethane-2,1-diyl))bis(oxy))bis(3,1-phenylene))bis(3-(3,4-dimethoxyphenyl)propane-1,1-diyl)(2s,2's)-bis(1-((s)-2-(3,4,5-trimethoxyphenyl)butanoyl)piperidine-2-carboxylate)
33. 2-piperidinecarboxylic Acid, 1-(1-oxo-2-(3,4,5-trimethoxyphenyl)butyl)-, 1,2-ethanediylbis(imino(2-oxo-2,1-ethanediyl)oxy-3,1-phenylene(3-(3,4-dimethoxyphenyl)propylidene)) Ester, (2s-(1(r*),2r*(s*(s*(1(r*),2r*)))))-
| Molecular Weight | 1411.6 g/mol |
|---|---|
| Molecular Formula | C78H98N4O20 |
| XLogP3 | 11.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 20 |
| Rotatable Bond Count | 39 |
| Exact Mass | 1410.67744153 g/mol |
| Monoisotopic Mass | 1410.67744153 g/mol |
| Topological Polar Surface Area | 262 Ų |
| Heavy Atom Count | 102 |
| Formal Charge | 0 |
| Complexity | 2330 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in bone marrow transplant and graft versus host disease.
Treatment of graft versus host disease
Rimiducis is used to activate inducible caspase-9 produced by a modified gene included in some CAR T-cell therapies. This activation produces rapid induction of apoptosis in activated modified T-cells and resolution of the signs and symptoms of graft versus host disease within 24 hours.
Rimiducid binds to a drug binding domain derived from human FK506-binding protein which is present on a modified form of inducible caspase-9. This binding results in dimerization and subsequent activation of caspase-9. This system was designed to function as a "safety switch" in CAR T-cell therapy used in hematological cancers. Retroviral vectors used in production of these modified cells preferentially integrate this gene nearby promoters associated with T-cell activation. This results in higher expression of the modified inducible caspase-9 product in activated T-cells. In practice, this allows for specific targeting of these active T-cells by rimiducid which results in a decrease in circulating cell numbers of over 90% in the setting of graft versus host disease. This specificity spares non-alloreactive T-cells and allows for successful reconstitution of the transplanted immune system from these cells.[24753538] Additionally, these non-alloreactive cells retain their sensitivity to rimiducid.


