

X


API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
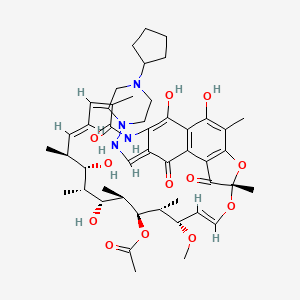

| Molecular Weight | 877.0 g/mol |
|---|---|
| Molecular Formula | C47H64N4O12 |
| XLogP3 | 5.8 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 6 |
| Exact Mass | 876.45207349 g/mol |
| Monoisotopic Mass | 876.45207349 g/mol |
| Topological Polar Surface Area | 217 A^2 |
| Heavy Atom Count | 63 |
| Formal Charge | 0 |
| Complexity | 1870 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Priftin |
| PubMed Health | Rifapentine (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | PRIFTIN (rifapentine) for oral administration contains 150 mg of the active ingredient rifapentine per tablet. The 150 mg tablets also contain, as inactive ingredients: calcium stearate, disodium EDTA, FD&C Blue No. 2 aluminum lake, hydroxypropyl cel. |
| Active Ingredient | Rifapentine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 2 of 2 | |
|---|---|
| Drug Name | Priftin |
| PubMed Health | Rifapentine (By mouth) |
| Drug Classes | Antitubercular |
| Drug Label | PRIFTIN (rifapentine) for oral administration contains 150 mg of the active ingredient rifapentine per tablet. The 150 mg tablets also contain, as inactive ingredients: calcium stearate, disodium EDTA, FD&C Blue No. 2 aluminum lake, hydroxypropyl cel. |
| Active Ingredient | Rifapentine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 150mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |






