


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
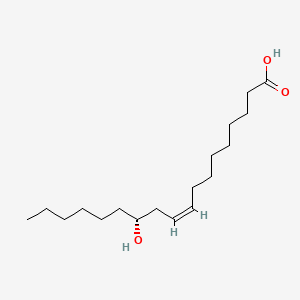

1. 12-d-hydroxy-9-trans-octadecenoic Acid
2. 12-hydroxy-9-octadecenic Acid
3. 12-hydroxy-9-octadecenoic Acid
4. 12-hydroxyoctadec-cis-9-enoic Acid
5. 9-octadecenoic Acid, 12-hydroxy-, (9e,12r)-
6. Ricinelaidic Acid
7. Ricinoleic Acid, (r-(e))-isomer
1. 141-22-0
2. Ricinolic Acid
3. Ricinic Acid
4. (r,z)-12-hydroxyoctadec-9-enoic Acid
5. Castor Oil Acid
6. 12-hydroxy-cis-9-octadecenoic Acid
7. D-12-hydroxyoleic Acid
8. (9z,12r)-12-hydroxyoctadec-9-enoic Acid
9. Kyselina Ricinolova
10. Oleic Acid, 12-hydroxy-
11. Nouracid Cs 80
12. 9-octadecenoic Acid, 12-hydroxy-, [r-(z)]-
13. Kyselina 12-hydroxy-9-oktadecenova
14. L'acide Ricinoleique
15. 12-hydroxyoleic Acid
16. Nsc 281242
17. 9-octadecenoic Acid, 12-hydroxy-, (9z,12r)-
18. Chebi:28592
19. 12-hydroxyoctadeca-9-enoic Acid
20. 12r-hydroxy-9z-octadecenoic Acid
21. 12-d-hydroxy-9-cis-octadecenoic Acid
22. I2d0f69854
23. 12-hydroxy-9-octadecenoic Acid
24. (r)-12-hydroxy-cis-9-octadecenoic Acid
25. Fatty Acids, Castor-oil
26. 9-octadecenoic Acid, 12-hydroxy-, (r-(z))-
27. Dsstox_cid_21567
28. Dsstox_rid_79777
29. Dsstox_gsid_41567
30. Riconoleic Acid
31. 61789-44-4
32. Ricinoleic Acid (purity Inverted Exclamation Marky99%)
33. Ricinusoleic Acid
34. Acide Ricinoleique
35. [r]-12-hydroxy-cis-9-octadecenoic Acid
36. Cas-141-22-0
37. Ncgc00161336-02
38. (cis,r)-12-hydroxyoctadec-9-enoic Acid
39. Acide Ricinoleique [french]
40. Kyselina Ricinolova [czech]
41. Ricinolsaeure
42. Ricinoleic-acid
43. 9-octadecenoic Acid, 12-hydroxy-
44. L'acide Ricinoleique [french]
45. Unii-i2d0f69854
46. Hsdb 5634
47. Nsc-281242
48. 9-octadecenoic Acid,12-hydroxy-,(z)-
49. Ncgc00181322-01
50. Einecs 205-470-2
51. Mfcd00084840
52. Vespula Pensylvanica B708568k062
53. (9z,12r)-12-hydroxy-9-octadecensaeure
54. (r-(z))-12-hydroxy-9-octadecenoic Acid
55. (z,12r)-12-hydroxyoctadec-9-enoic Acid
56. 9-octadecenoic Acid, 12-hydroxy-, (z)-
57. Ai3-17956
58. (9z,12r)-12-hydroxy-9-octadecenoic Acid
59. Unii-kd8t3w6vzx
60. 9-octadecenoic Acid, 12-hydroxy-, (cis)-
61. Kyselina 12-hydroxy-9-oktadecenova [czech]
62. Kd8t3w6vzx
63. Ricinoleic Acid, >=99%
64. Schembl18144
65. Flexricin 100 (salt/mix)
66. Ricinoleic Acid [mi]
67. Ricinoleic Acid [hsdb]
68. Ricinoleic Acid [inci]
69. Bml2-f10
70. Ricinoleic Acid [vandf]
71. 9-octadecenoicacid,12-hydroxy-
72. Chembl3186422
73. Dtxsid0041567
74. Ricinoleic Acid [mart.]
75. Hy-n6070a
76. Ricinoleic Acid [who-dd]
77. Hy-n6070
78. 12-hydroxy-9(z)-octadecenoic Acid
79. Ricinoleic Acid, Analytical Standard
80. Tox21_113406
81. Tox21_301098
82. Lmfa02000184
83. Zinc12954494
84. 12r-hydroxy-9-cis-octadecenoic Acid
85. Akos016005826
86. (9z)-(12r)-hydroxyoctadecenoic Acid
87. Db02955
88. (z,r)-12-hydroxyoctadec-9-enoic Acid
89. 12-oh 9c-18:1
90. Ncgc00161336-01
91. Ncgc00161336-03
92. Ncgc00181089-01
93. Ncgc00254998-01
94. (9z)-12-hydroxy-9-octadecenoic Acid #
95. As-65782
96. Cs-0032307
97. Cs-0204122
98. R0027
99. C08365
100. Ricinoleic Acid, Vetec(tm) Reagent Grade, 80%
101. A885691
102. Q424484
103. 9-octadecenoic Acid, 12-hydroxy-, (theta-(z))-
104. W-108189
| Molecular Weight | 298.5 g/mol |
|---|---|
| Molecular Formula | C18H34O3 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 15 |
| Exact Mass | 298.25079494 g/mol |
| Monoisotopic Mass | 298.25079494 g/mol |
| Topological Polar Surface Area | 57.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 261 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
2. 2= Slightly toxic, Probable oral lethal dose (human) is 5-15 g/kg, between 1 pint and 1 quart for 70 kg person (150 lb).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-134
The lowest dose at which mortality occurred following an accidental ingestion was 5000 mg/kg.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 4967
Ricinoleic acid or methyl ricinoleate was administered via gastric intubation to male rats (minimum weight of 400 g) with a cannulated thoracic duct. Lymph was collected for 48 hr, and the lipids were then extracted and separated into various lipid classes. Results indicated that Ricinoleic acid was present in the triglyceride, diglyceride, monoglyceride, and free fatty acid fractions. Peak absorption of ricinoleic acid occurrred within 30 min post administration. Ricinoleic acid was not present in the phospholipid or cholesterol ester fractions of the lymph lipids.
Cosmetic Ingredient Expert Review Panel; Final Report on the Safety Assessment of Ricinus Communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate; International Journal of Toxicology 26 (3 Suppl): 31-77 (2007).
The skin penetration of ricinoleic acid in vivo /was investigated/ using rats that were 20 to 30 days old. In order to increase the fluorescence of ricinoleic acid, either one part of methyl anthranilate or methylcholanthrene was added to 99 parts ricinoleic acid. The test substance was gently rubbed on to skin that had been clipped free of hair. Biopsies were taken at various intervals post application. The preparations were observed using a Spencer microscope with a quartz condenser. Ricinoleic acid was retained mainly in the outer strata of the epidermis. There was little evidence of deeper penetration in biopsies that were taken at 2 hr post application.
Cosmetic Ingredient Expert Review Panel; Final Report on the Safety Assessment of Ricinus Communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate; International Journal of Toxicology 26 (3 Suppl): 31-77 (2007).
The percutaneous absorption of a radiolabeled (3H)ricinoleic acid (specific activity = 20.0 mCi/mmol) mixture was evaluated using porcine skin membranes or silastic (polydimethylsioloxane) membranes in the Bronaugh flow-through diffusion cell system. [3H]Ricinoleic acid (5%) mixtures were prepared in water containing either 5% mineral oil or 5% PEG 200. Other (3H)Ricinoleic acid mixtures were formulated with the following three commonly used cutting fluid additives: triazine, linear alkylbenzene sulfonate, and triethanolamine. At 8 hr after topical exposure, Ricinoleic acid absorption (based on amount recovered in receptor fluid) ranged from 1% to 13% in silastic membranes and 0.1% to 0.3% in porcine skin membranes. For most mixtures, peak absorption of ricinoleic acid occurred within 3 hr. The greatest ricinoleic acid peak concentrations were associated with the control mixtures containing PEG in both membranes.
Cosmetic Ingredient Expert Review Panel; Final Report on the Safety Assessment of Ricinus Communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate; International Journal of Toxicology 26 (3 Suppl): 31-77 (2007).
/Investigators/ studied the accumulation of hydroxyl acids in depot fat after rats were fed with ricinoleic acid in two experiments. In the first experiment, adult male rats (number, strain, and weights not stated) were fed ricinoleic acid (5% emulsion, 20 mL) for 7 days. In the second experiment, the animals were fed for 27 days. Lipid extraction from the fat tissue was followed by hydrolysis to yield a fatty acid mixture. A gas-liquid chromatogram indicated appreciable amounts of the following hydroxy fatty acids with shorter chain lengths than ricinoleic acid: 10-hydroxyhexadecenoic acid (experiment 1: 0.60% of total fatty acids; experiment 2: 0.33% of total fatty acids), 8-hydroxytetradecenoic acid (experiment 1: 0.03% of total fatty acids; experiment 2: 0.08% of total fatty acids), and 6-hydroxydodecenoic acid (experiment 2: 0.03% of total fatty acids). Ricinoleic acid comprised 0.51% of total fatty acids in experiment 1 and 3.85% of total fatty acids in experiment 2.
Cosmetic Ingredient Expert Review Panel; Final Report on the Safety Assessment of Ricinus Communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate; International Journal of Toxicology 26 (3 Suppl): 31-77 (2007).
For more Absorption, Distribution and Excretion (Complete) data for Ricinoleic acid (9 total), please visit the HSDB record page.
Three healthy subjects /were/ administered castor oil (10 to 15 mL) orally. Urine was collected between 2 and 8 hr post dosing. The following three epoxydicarboxylic acids were excreted in the urine: 3,6-epoxyoctanedioic acid; 3,6-epoxydecanedioic acid; and 3,6-epoxydodecanedioic acid. These three ricinoleic acid metabolites were also detected in the urine of rats...
Cosmetic Ingredient Expert Review Panel; Final Report on the Safety Assessment of Ricinus Communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate; International Journal of Toxicology 26 (3 Suppl): 31-77 (2007).


