


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
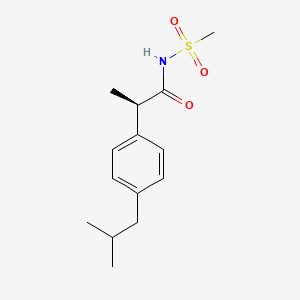

1. (r)-2-(4-isobutylphenyl)propionylmethanesulfonamide
2. 2-(4-isobutylphenyl)propionylmethanesulfonamide
3. Df 1681y
4. Df-1681y
5. Df1681y
6. Reparixin Lysine
7. Reparixin Lysine Salt
8. Repertaxin
1. Repertaxin
2. 266359-83-5
3. Df 1681y
4. Df1681y
5. Reparixin (repertaxin)
6. (r)-2-(4-isobutylphenyl)-n-(methylsulfonyl)propanamide
7. Df-1681y
8. Chembl191413
9. U604e1nb3k
10. 266359-83-5 (free Base)
11. Reparixin [inn]
12. (2r)-2-[4-(2-methylpropyl)phenyl]-n-methylsulfonylpropanamide
13. Reparixin [usan:inn]
14. Unii-u604e1nb3k
15. (2r)-2-(4-(2-methylpropyl)phenyl)-n-methylsulfonylpropanamide
16. 2-(4-isobutylphenyl)propionylmethanesulfonamide
17. Reparixin (usan/inn)
18. Reparixin [usan]
19. Reparixin [mart.]
20. Reparixin [who-dd]
21. Dsstox_cid_26509
22. Dsstox_rid_81678
23. Dsstox_gsid_46509
24. Gtpl8498
25. Schembl1884299
26. Zinc8717
27. Dtxsid6046509
28. Chebi:177765
29. Bcp10635
30. Ex-a2461
31. Tox21_112272
32. Bdbm50169045
33. Mfcd18633292
34. S8640
35. Ccg-267282
36. Cs-1379
37. Db12614
38. Benzeneacetamide, Alpha-methyl-4-(2-methylpropyl)-n-(methylsulfonyl)-, (alphar)-
39. Ac-32023
40. Bs-16754
41. Hy-15251
42. Cas-266359-83-5
43. D08984
44. Q27088533
45. (2r)-2-[4-(2-methylpropyl)phenyl]-n-methylsulonylpropanamide
46. N-[(r)-2-(4-isobutyl-phenyl)-propionyl]-methanesulfonamide
47. Benzeneacetamide, Alpha-methyl-4-(2-methylpropyl)-n-(methylsulfonyl)- (alphar)-
48. Benzeneacetamide, .alpha.-methyl-4-(2-methylpropyl)-n-(methylsulfonyl)-, (.alpha.r)-
| Molecular Weight | 283.39 g/mol |
|---|---|
| Molecular Formula | C14H21NO3S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 283.12421471 g/mol |
| Monoisotopic Mass | 283.12421471 g/mol |
| Topological Polar Surface Area | 71.6 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 389 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Prevention of graft rejection
Treatment of coronavirus disease 2019 (COVID-2019)


