


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
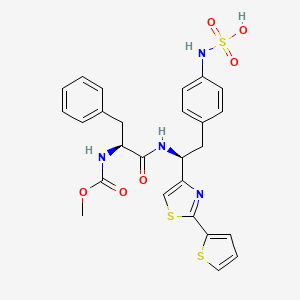

1. Akb-9778
1. 1008510-37-9
2. Akb-9778
3. Razuprotafib [inn]
4. Razuprotafib [usan]
5. 0wax4ut396
6. Razuprotafib (usan)
7. [4-[(2s)-2-[[(2s)-2-(methoxycarbonylamino)-3-phenylpropanoyl]amino]-2-(2-thiophen-2-yl-1,3-thiazol-4-yl)ethyl]phenyl]sulfamic Acid
8. Carbamic Acid, N-((1s)-2-oxo-1-(phenylmethyl)-2-(((1s)-2-(4-(sulfoamino)phenyl)-1-(2-(2-thienyl)-4-thiazolyl)ethyl)amino)ethyl)-, C-methyl Ester
9. N-(4-{(2s)-2-{(2s)-2-[(methoxycarbonyl)amino]-3-phenylpropanamido}-2-[2-(thiophen-2-yl)-1,3-thiazol-4-yl]ethyl}phenyl)sulfamic Acid
10. Razuprotafib [usan:inn]
11. Unii-0wax4ut396
12. Razuprotafib [who-dd]
13. Schembl679459
14. Chembl3931971
15. Gtpl11336
16. Akb9778
17. Bdbm359124
18. Us10220048, Compound Aa34
19. Who 10271
20. Compound Aa34 [us10220048]
21. At18581
22. Hy-109041
23. Cs-0031483
24. D11540
25. N-(4-((2s)-2-((2s)-2-((methoxycarbonyl)amino)- 3-phenylpropanamido)-2-(2-(thiophen-2-yl)-1,3-thiazol4-yl)ethyl)phenyl)sulfamic Acid
| Molecular Weight | 586.7 g/mol |
|---|---|
| Molecular Formula | C26H26N4O6S3 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 12 |
| Exact Mass | 586.10144808 g/mol |
| Monoisotopic Mass | 586.10144808 g/mol |
| Topological Polar Surface Area | 212 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 906 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Razuprotafib inhibits [VE-PTP](https://go.drugbank.com/bio_entities/BE0003769) (a negative regulator of Tie2 in diseased blood vessels) by binding and inhibiting the intracellular catalytic domain of VE-PTP that inactivates Tie2. This in turn allows razuprotafib to restore Tie2 activation to allow for enhancement of endothelial function and stabilization of blood vessels. Razuprotafib is being investigated against diabetic vascular complications and acute respiratory distress syndrome (ARDS) in COVID-19.


