



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
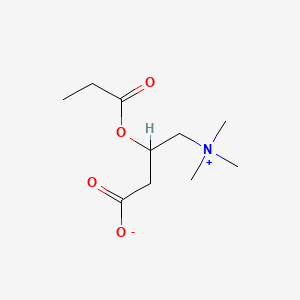

1. L-propionylcarnitine
2. Propionyl Carnitine
3. Propionylcarnitine, (+-)-isomer
4. Propionylcarnitine, (r)-isomer
1. Propionyl Carnitine
2. 17298-37-2
3. O-propionylcarnitine
4. 3-(propionyloxy)-4-(trimethylammonio)butanoate
5. 3-propanoyloxy-4-(trimethylazaniumyl)butanoate
6. 3-(propanoyloxy)-4-(trimethylazaniumyl)butanoate
7. Chebi:28867
8. 3-carboxy-n,n,n-trimethyl-2-(1-oxopropoxy)-1-propanaminium Inner Salt
9. 1-propanaminium, 3-carboxy-n,n,n-trimethyl-2-(1-oxopropoxy)-, Inner Salt
10. Hsdb 7589
11. Acylcarnitine C3:0
12. Schembl156850
13. Chembl2074916
14. Dtxsid20938255
15. Lmfa07070105
16. O-propanoylcarnitine (internal Charge)
17. Hy-113092
18. Cs-0059547
19. C03017
20. (3-carboxy-2-propanoyloxypropyl)-trimethylazanium
21. Q27103933
| Molecular Weight | 217.26 g/mol |
|---|---|
| Molecular Formula | C10H19NO4 |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 217.13140809 g/mol |
| Monoisotopic Mass | 217.13140809 g/mol |
| Topological Polar Surface Area | 66.4 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 227 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
L-Carnitine, acetyl-L-carnitine, and/or propionyl-L-carnitine may be used for replacement therapy to restore normal carnitine concn and/or a normal nonesterified-to-esterified carnitine ratio ...
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 76 (2005)
/EXPL THER/ The aim of this double-blind, placebo-controlled, dose titration, multicenter trial was to assess the efficacy and safety of propionyl-carnitine in intermittent claudication. ... After a 2-week preliminary period to assess maximal walking distance, 245 patients were randomly assigned to receive propionyl-L-carnitine (n = 118) or placebo (n = 127). The initial oral dose of 500 mg twice daily was increased at 2-month intervals to 2 g/day and then to 3 g/day in patients showing improvement in treadmill performance < 30% over baseline. Efficacy analysis was conducted for the 214 patients who completed the 24 weeks of treatment by comparing the effect of placebo and propionyl-L-carnitine on day 180. ... Analysis of variance showed a significant improvement of 73 +/- 9% (mean +/- SE) in maximal walking distance with propionyl-L-carnitine (n = 99) compared with 46 +/- 6% for placebo (n = 115, p = 0.03). For distance walked at onset of claudication, propionyl-L-carnitine showed about double the improvement of placebo; however, the difference was not statistically significant. There were no changes in electrocardiographic and routine biochemical and hematologic tests that would indicate an adverse effect of propionyl-L-carnitine. Adverse events requiring drug discontinuation (11 in the propionyl-L-carnitine group, 3 in the placebo group) were unrelated to study medication. The dose titration design of the study also provided information on the dose-response relation. Slightly less than 67% of patients were expected to improve their maximal walking distance by at least 30%, assuming 2 g/day of propionyl-L-carnitine (95% confidence interval 0.51 to 0.70). The response rate during the entire titration course was significantly in favor of propionyl-L-carnitine compared with placebo. ...
PMID:7594063 Brevetti G et al; J Am Coll Cardiol 26 (6):1411-6 (1995)
/EXPL THER/ Propionyl-l-carnitine (PLC) is a naturally occurring compound that has been considered for the treatment of many forms of cardiomyopathies.
PMID:11419959 Sayed-Ahmed MM et al; Pharmacol Res 43(6): 513-20 (2001)
/EXPL THER/ Propionyl-L-carnitine is a carnitine derivative that has a high affinity for muscular carnitine transferase, and it increases cellular carnitine content, thereby allowing free fatty acid transport into the mitochondria. ... The results of phase-2 studies in chronic heart failure patients showed that long-term oral treatment with propionyl-L-carnitine improves maximum exercise duration and maximum oxygen consumption over placebo and indicated a specific propionyl-L-carnitine effect on peripheral muscle metabolism. A multicenter trial on 537 patients showed that propionyl-L-carnitine improves exercise capacity in patients with heart failure, but preserved cardiac function.
PMID:15591005 Ferrari R et al; Ann N Y Acad Sci 1033:79-91 (2004)
For more Therapeutic Uses (Complete) data for PROPIONYL-L-CARNITINE (13 total), please visit the HSDB record page.
Propionyl-L-carnitine stimulates a better efficiency of the Krebs cycle during hypoxia by providing it with a very easily usable substrate, propionate, which is rapidly transformed into succinate without energy consumption (anaplerotic pathway). Alone, propionate cannot be administered to patients in view of its toxicity.
PMID:15591005 Ferrari R et al; Ann N Y Acad Sci 1033:79-91 (2004)
... the efficacy and safety of oral vitamin E and propionyl-L-carnitine, separately or in combination, /were compared/ for the treatment of Peyronie's disease. ... A total of 236 men (mean age 43.4 years) with Peyronie's disease were randomly assigned to 4 groups. Group 1 (58 men) received 300 mg vitamin E orally twice daily. Group 2 (59) received 1 gm propionyl-L-carnitine orally twice daily, and group 3 (60) received 300 mg vitamin E and 1 gm propionyl-L-carnitine orally twice daily. Group 4 (control group, 59 men) received a similar regimen of placebo during the 6-month treatment period. . ... This study did not show significant improvement in pain, curvature or plaque size in patients with PD treated with vitamin E, propionyl-L-carnitine, or vitamin E plus propionyl-L-carnitine compared with those treated with placebo. Publication
PMID:17706714 Safarinejad MR et al; J Urol 178 (4 Pt 1): 1398-403; discussion 1403 (2007)
Cardiotonic Agents
Agents that have a strengthening effect on the heart or that can increase cardiac output. They may be CARDIAC GLYCOSIDES; SYMPATHOMIMETICS; or other drugs. They are used after MYOCARDIAL INFARCT; CARDIAC SURGICAL PROCEDURES; in SHOCK; or in congestive heart failure (HEART FAILURE). (See all compounds classified as Cardiotonic Agents.)
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Most (54-86%) dietary carnitine is absorbed in the small intestine and enters the bloodstream. The kidneys efficiently conserve carnitine, so even carnitine-poor diets have little impact on the body's total carnitine content. Rather than being metabolized, excess carnitine is excreted in the urine as needed via the kidneys to maintain stable blood concentrations. /Carnitine/
NIH/ODS; Dietary Supplement Fact Sheets on Carnitine (6/15/2006). Available from, as of February 26, 2008: https://ods.od.nih.gov/factsheets/carnitine.asp
L-Carnitine and acylcarnitine esters are present in all tissues. In most tissues and cells, they are present in higher concn than in the circulation ... L-carnitine and acetyl-L-carnitine are concn in most tissues via the high-affinity, Na+-dependent organic cation transporter OCTN2 ... OCTN2 binds acetyl-L-carnitine and propionyl-L-carnitine with comparable affinity. This protein is highly expressed in heart, placenta, skeletal muscle, kidney, pancreas, testis, and epididymis and weakly expressed in brain, lung, and liver ... /Acylcarnitine esters/
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 75 (2005)
... At a filtered load of 50 umol/L, the efficiency of L-carnitine and acylcarnitine ester reabsorption is 90 to 98% /in kidneys/. However, as the filtered load of L-carnitine incr, as, eg after consumption of a dietary supplement or after iv infusion, the efficiency of reabsorption declines rapidly ... Clearance of acylcarnitine esters is often higher than that of nonesterified L-carnitine /in kidneys/ ... Under conditions of rapid intracellular synth of acylcarnitine esters or direct accumulation from the circulation ... a higher proportion of acylcarnitine esters in urine compared to that in the circulation /is achieved/ ... Kidneys may be substantially involved in the regulation of circulating acylcarnitine ester concn. /Aacylcarnitine ester/
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 76 (2005)
... A concn ratio of acylcarnitine esters/nonesterified L-carnitine of 0.4 or greater in plasma is ... considered abnormal ...
Coates, P.M., Blackman, M.R., Cragg, G.M., Levine, M., Moss, J., White, J.D. (Ed), Encyclopedia of Dietary Supplements. Marcel Dekker, New York, NY, p. 76 (2005)
L-Propionylcarnitine, a propionyl ester of L-carnitine, increases the intracellular pool of L-carnitine. It exhibits a high affinity for the enzyme carnitine acetyltransferase (CAT) and, thus, is readily converted into propionyl-coenzyme A and free carnitine. It has been reported that L-propionylcarnitine possesses a protective action against heart ischemia-reperfusion injury;... To obtain a better insight into the antiradical mechanism of L-propionylcarnitine, the present research analyzed the superoxide scavenging capacity of L-propionylcarnitine and its effect on linoleic acid peroxidation. In addition, the effect of L-propionylcarnitine against DNA cleavage was estimated using pBR322 plasmid. ... L-propionylcarnitine showed a dose-dependent free-radical scavenging activity. In fact, it was able to scavenge superoxide anion, to inhibit the lipoperoxidation of linoleic acid, and to protect pBR322 DNA from cleavage induced by H2O2 UV-photolysis.
PMID:10917565 Vanella A et al; Cell Biol Toxicol 16 (2): 99-104 (2000)


