



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Berlin Blue
2. Ferric Ferrocyanide
3. Ferrihexacyanoferrate
4. Ferrocin
5. Ferrotsin
1. Ferric Ferrocyanide
2. Iron(iii) Hexacyanoferrate(ii)
3. Prussian Blue Insoluble
4. Iron(2+);iron(3+);octadecacyanide
5. Iron(iii) Ferrocyanide; Milori Blue
6. Ferrocin
7. Parisian Blue
8. Preussischblau
9. Turnbulls Blau
10. Berliner Blau
11. Mfcd00135663
12. Iron (iii) Ferrocyanide
13. Chebi:30069
14. Iron(3+) Hexacyanoferrate(4-)
15. Iron(iii) Hexacyanidoferrate(ii)
16. Iron(3+) Hexacyanidoferrate(4-)
17. Akos025310680
18. Db06783
19. Q421894
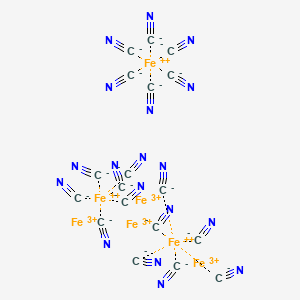
| Molecular Weight | 859.2 g/mol |
|---|---|
| Molecular Formula | C18Fe7N18 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 36 |
| Rotatable Bond Count | 0 |
| Exact Mass | 859.59988 g/mol |
| Monoisotopic Mass | 859.59988 g/mol |
| Topological Polar Surface Area | 428 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 127 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 25 |
Indicated for treatment of patients with known or suspected internal contamination with radioactive cesium and/or radioactive or non-radioactive thallium to increase their rates of elimination.
FDA Label
Prussian blue is an insoluble radioactive metals chelating agent and absorbent. It acts by ion-exchange, adsorption, and mechanical trapping within the crystal structure and has a very high affinity for radioactive and non-radioactive cesium and thallium. The antidote therapy greatly minimizes the extent of contamination and reduces the half life of radioactive isotopes which have relatively long physicall half life and uniform tissue distribution. Data suggest that in humans, Prussian blue can reduce cesiums half-life by approximately 43% and reduce total body burdens by significantly increasing the feces-to-urine excretion ratio.
Antidotes
Agents counteracting or neutralizing the action of POISONS. (See all compounds classified as Antidotes.)
Coloring Agents
Chemicals and substances that impart color including soluble dyes and insoluble pigments. They are used in INKS; PAINTS; and as INDICATORS AND REAGENTS. (See all compounds classified as Coloring Agents.)
V - Various
V03 - All other therapeutic products
V03A - All other therapeutic products
V03AB - Antidotes
V03AB31 - Prussian blue
Absorption
It is poorly or not absorbed from the gastrointestinal tract walls after oral ingestion. Systemic absorption is assumed to be insignificant, with minimal release of cyanide from the complex. A small amount (approximately 2%) of the hexacyanoferrate ion was absorbed after oral ingestion of prussian blue but with no signs of decomposition. Prussian blue is not systemically bioavailable.
Route of Elimination
It predominantly depends on fecal excretion, and does not depend on renal elimination. Based on animal data, 99% of a single dose of 40 mg of labeled insoluble Prussian blue was excreted unchanged in feces.
Volume of Distribution
Histopathological examination of different organs showed no deposits of prussian blue after oral administration of insoluble prussian blue.
Clearance
The clearance from the body depends on the gastrointestinal tract transit time.
No evidence of decomposition after oral ingestion. Prussian blue does not undergo hepatic metabolism; use of the drug is not contraindicated in patients with hepatic impairment.
Prussian blue binds cesium and thallium isotopes in the gastrointestinal tract after ingestion or excreted in the bile by the liver, therby reduces gastrointestinal reabsorption into the enterohepatic circulation. It serves as an ion exchanger for univalent cations and it preferentially binds to cesium or thallium as its affinity for cations increases as the ionic radius of the cation increases. Prussian blue exchanges potassium for cesium or thallium at the surface of the crystal in the intestinal lumen. The insoluble complex is excreted without being absorbed from the intestinal walls. Insoluble prussian blue decreases the half life of cesium by 33% and from 3.8 to 2.2 days for thallium. The rate of cesium and thallium elimination is proportional to the dose and duration of prussian blue.






