



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
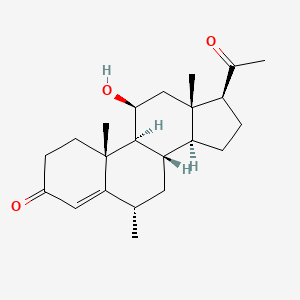

1. 11-hydroxy-6-methylprogesterone
2. Hms Liquifilm
3. Hms Suspension
1. 2668-66-8
2. Hydroxymesterone
3. Medrifar
4. Medrocort
5. Medritonic
6. Visudrisone
7. Hms Liquifilm
8. 6alpha-methyl-11beta-hydroxyprogesterone
9. U-8471
10. Nsc-63278
11. Medrysone (usan)
12. 11beta-hydroxy-6alpha-methylpregn-4-ene-3,20-dione
13. Gsh 1043
14. (6s,8s,9s,10r,11s,13s,14s,17s)-17-acetyl-11-hydroxy-6,10,13-trimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one
15. D2ufc189xf
16. Mls000028714
17. Chebi:34829
18. U 8471
19. 11.beta.-hydroxy-6.alpha.-methylpregn-4-ene-3,20-dione
20. Smr000058693
21. Medrysone [usan]
22. Dsstox_cid_25371
23. Dsstox_rid_80833
24. Dsstox_gsid_45371
25. Medriabioptal
26. Medrysonum
27. Sedesterol
28. Spectramedryn
29. Medrisona
30. Medrisone
31. Medriusar
32. Medroftal
33. Medrisone [dcit]
34. Medrysonum [inn-latin]
35. Medrisona [inn-spanish]
36. Unii-d2ufc189xf
37. Medrysone [usan:usp:inn]
38. Prestwick_89
39. Ncgc00016616-01
40. Cas-2668-66-8
41. Einecs 220-208-7
42. Nsc 63278
43. 11beta-hydroxy-6alpha-methylprogesterone
44. Cpd000058693
45. Medrysone [inn]
46. Opera_id_635
47. Medrysone [mi]
48. 6-alpha-methyl-11-beta-hydroxyprogesterone
49. Prestwick0_000743
50. Prestwick1_000743
51. Prestwick2_000743
52. Prestwick3_000743
53. Medrysone [vandf]
54. Progesterone, 11beta-hydroxy-6alpha-methyl-
55. 11.beta.-hydroxy-6.alpha.-methylprogesterone
56. 6.alpha.-methyl-11.beta.-hydroxyprogesterone
57. Medrysone [mart.]
58. Hms (tn)
59. Medrysone [who-dd]
60. Schembl4493
61. Bspbio_000726
62. Mls001076155
63. Mls002207230
64. Mls002222162
65. Spbio_002665
66. Bpbio1_000800
67. Gtpl7086
68. Medrysone [orange Book]
69. 11-beta-hydroxy-6-alpha-methylpregn-4-ene-3,20-dione
70. Chembl1201173
71. Dtxsid6045371
72. 6-methyl-11-hydroxyprogesterone
73. Bcbcmap01_000088
74. 6a-methyl-11b-hydroxyprogesterone
75. Hms1570e08
76. Hms2097e08
77. Hms2232f13
78. Hms3259a22
79. Hms3714e08
80. Caa66866
81. Hy-b1076
82. Nsc63278
83. Zinc3977945
84. Tox21_110526
85. Akos024285179
86. Pregn-4-ene-3,20-dione, 11-hydroxy-6-methyl-, (6.alpha.,11.beta.)-
87. Tox21_110526_1
88. Ccg-220743
89. Cs-4632
90. Db00253
91. Nc00599
92. Smp1_000061
93. 6+/--methyl-11(2)-hydroxyprogesterone
94. Ncgc00179460-01
95. Ncgc00179460-03
96. D02289
97. 668m668
98. Progesterone, 11.beta.-hydroxy-6.alpha.-methyl-
99. Sr-01000721900
100. Q6807292
101. Sr-01000721900-3
102. Brd-k56515112-001-03-4
103. Brd-k56515112-001-21-6
104. Pregn-4-ene-3, 11.beta.-hydroxy-6.alpha.-methyl-
105. Wln: L E5 B666 Ov Mutj A1 Cq E1 Fv1 L1
106. Pregn-4-ene-3,20-dione, 11beta-hydroxy-6alpha-methyl-
107. Pregn-4-ene-3,20-dione, 11-beta-hydroxy-6-alpha-methyl-
108. Medrysone, United States Pharmacopeia (usp) Reference Standard
109. Pregn-4-ene-3, 11-hydroxy-6-methyl-, (6.alpha.,11.beta.)-
110. Pregn-4-ene-3,20-dione, 11-hydroxy-6-methyl-, (6alpha,11beta)-
111. (1s,2r,8s,10s,11s,14s,15s,17s)-14-acetyl-17-hydroxy-2,8,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one
112. (6s,10r,11s,13s,17s)-17-acetyl-11-hydroxy-6,10,13-trimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one
| Molecular Weight | 344.5 g/mol |
|---|---|
| Molecular Formula | C22H32O3 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 344.23514488 g/mol |
| Monoisotopic Mass | 344.23514488 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 650 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of allergic conjunctivitis, vernal conjunctivitis, episcleritis, and epinephrine sensitivity.
FDA Label
Medrysone is a topical anti-inflammatory corticoidsteroids for ophthalmic use. In patients with increased intraocular pressure and in those susceptible to a rise in intraocular pressure, there is less effect on pressure with medrysone than with dexamethasone or betamethasone. Corticoidsteroids inhibit the edema, fibrin deposition, capillary dilation, and phagocytic migration of the acute inflammatory response, as well as capillary proliferation, deposition of collagen, and scar formation.
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BA - Corticosteroids, plain
S01BA08 - Medrysone
Absorption
Rapidly absorbed following oral administration.
There is no generally accepted explanation for the mechanism of action of ocular corticosteroids. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2. Initially, the drug binds to the glucocorticoid receptor in the cytosol. This migrates to the nucleus and binds to genetic elements which cause activation and repression of the involved genes in the inflammatory pathway.


