



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
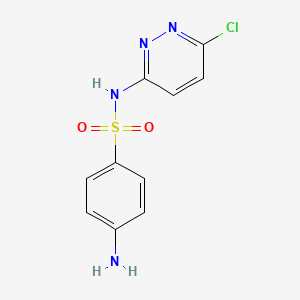

1. Monosodium Salt Sulfachlorpyridazine
2. Sulfachloropyridazine
3. Sulfachlorpyridazine, Monosodium Salt
4. Sulphachlorpyridazine
1. Sulfachloropyridazine
2. 80-32-0
3. 4-amino-n-(6-chloropyridazin-3-yl)benzenesulfonamide
4. Sulphachlorpyridazine
5. Nefrosul
6. Sonilyn
7. N1-(6-chloro-3-pyridazinyl)sulfanilamide
8. 4-amino-n-(6-chloro-3-pyridazinyl)benzenesulfonamide
9. Sulfacloropiridazina
10. Durasulf
11. Sulfachlorpyridazinum
12. Vetisulid
13. Benzenesulfonamide, 4-amino-n-(6-chloro-3-pyridazinyl)-
14. Ba 10370
15. Sulfachloropyridizine
16. Chebi:59057
17. Nsc-757858
18. Mls000777734
19. N(sup 1)-(6-chloro-3-pyridazinyl)sulfanilamide
20. P78d9p90c0
21. 4-amino-n-(6-chloropyridazin-3-yl)-benzenesulfonamide
22. Cas-80-32-0
23. Ncgc00016323-01
24. Ncgc00016323-04
25. Cluricol
26. Smr000414174
27. N(1)-(6-chloro-3-pyridazinyl)sulfanilamide
28. Dsstox_cid_25265
29. Dsstox_rid_80778
30. Dsstox_gsid_45265
31. Sulfachloropyridazine 100 Microg/ml In Acetonitrile
32. 4-amino-n-(6-chloro-pyridazin-3-yl)-benzenesulfonamide
33. Vetisulid (veterinary)
34. Prinzone Vet.
35. Solfaclorpiridazina
36. Solfaclorpiridazina [dcit]
37. Scp
38. Sulphachloropyridazine
39. Sulfachlorpyridazinum [inn-latin]
40. Sr-01000773478
41. Sulfacloropiridazina [inn-spanish]
42. Einecs 201-269-9
43. N1-(6-chlor-3-pyridazinyl)sulfanilamid
44. Cosulid
45. Unii-p78d9p90c0
46. Nefrosul (tn)
47. Prestwick_130
48. Sulfachlorpyridazine [usp:inn:ban]
49. Mfcd00057371
50. Cosumix (salt/mix)
51. 4-amino-n-(6-chloropyridazin-3-yl)benzene-1-sulfonamide
52. Spectrum_000127
53. Prestwick0_000715
54. Prestwick1_000715
55. Prestwick2_000715
56. Prestwick3_000715
57. Spectrum2_001942
58. Spectrum3_001221
59. Spectrum4_000444
60. Spectrum5_001011
61. Epitope Id:122243
62. Cid_6634
63. Ciba 10370
64. Oprea1_485631
65. Schembl94138
66. Bspbio_000929
67. Bspbio_002662
68. Kbiogr_000828
69. Kbioss_000587
70. Spectrum1501142
71. Spbio_002003
72. Spbio_002850
73. Bpbio1_001023
74. Sulfachlorpyridazine (usp/inn)
75. [(4-aminophenyl)sulfonyl](6-chloropyridazin-3-yl)amine
76. Chembl1443577
77. Dtxsid9045265
78. Bdbm90673
79. Kbio2_000587
80. Kbio2_003155
81. Kbio2_005723
82. Kbio3_002162
83. Sulfachlorpyridazine [mi]
84. Zinc49140
85. Sulfachlorpyridazine [inn]
86. Hms1570o11
87. Hms1921j11
88. Hms2092f19
89. Hms2097o11
90. Hms2766b06
91. Hms3714o11
92. Pharmakon1600-01501142
93. Albb-011657
94. Hy-b1781
95. Str03778
96. Sulfachlorpyridazine [mart.]
97. Tox21_110372
98. Tox21_112957
99. Bbl037369
100. Ccg-39459
101. Nsc757858
102. S3708
103. Stk315422
104. Sulfachlorpyridazine [usp-rs]
105. Sulfachlorpyridazine [who-dd]
106. Akos000308728
107. Tox21_110372_1
108. Cs-7970
109. Db11461
110. Nsc 757858
111. Ncgc00016323-02
112. Ncgc00016323-03
113. Ncgc00016323-09
114. Ncgc00094909-01
115. Ncgc00094909-02
116. Sulfachlorpyridazine [green Book]
117. Ac-12002
118. N'-(6-chloro-3-pyridazinyl)sulfanilamide
119. Sbi-0051655.p002
120. Sulfachlorpyridazine [usp Monograph]
121. Ab00052217
122. Ft-0674699
123. C76571
124. D05948
125. Ab00052217_11
126. Sulfachloropyridazine 100 Microg/ml In Methanol
127. A839891
128. Sulfachloropyridazine 1000 Microg/ml In Methanol
129. Sr-01000773478-2
130. Sr-01000773478-3
131. W-104238
132. 4-amino-n-(6-chloro-3-pyridazinyl)-benzenesulfnamide
133. Brd-k32021043-001-05-1
134. Q27126423
135. 4-amino-n-(6-chloro-3-pyridazinyl)benzenesulfonamide #
136. 4-azanyl-n-(6-chloranylpyridazin-3-yl)benzenesulfonamide
137. Pyridazin-3-amine, N-(4-aminophenylsulfonyl)-6-chloro-
138. Sulfachloropyridazine, Vetranal(tm), Analytical Standard
139. Sulfachlorpyridazine, United States Pharmacopeia (usp) Reference Standard
140. N'-[(5z)-7,7-dimethyl-3-oxo-2,6,7,8-tetrahydrocinnolin-5(3h)-ylidene]-n,n-dimethylhydrazonoformamide
| Molecular Weight | 284.72 g/mol |
|---|---|
| Molecular Formula | C10H9ClN4O2S |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 284.0134744 g/mol |
| Monoisotopic Mass | 284.0134744 g/mol |
| Topological Polar Surface Area | 106 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 365 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents, Urinary
Substances capable of killing agents causing urinary tract infections or of preventing them from spreading. (See all compounds classified as Anti-Infective Agents, Urinary.)
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)


