



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
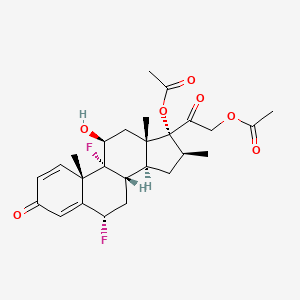

1. Diflorasone
2. Florone
3. Flutone
4. Maxiflor
5. Psorcon
6. Psorcon E
1. 33564-31-7
2. Psorcon
3. Florone E
4. Maxiflor
5. Apexicon E
6. Diflorasone Di(acetate)
7. 7w2j09scwx
8. Mls000069559
9. Mls001076548
10. [2-[(6s,8s,9r,10s,11s,13s,14s,16s,17r)-17-acetyloxy-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Acetate
11. 6alpha,9-difluoro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-diacetate
12. Chebi:31483
13. U-34865
14. U-34,865
15. Ncgc00022003-03
16. Smr000058814
17. Psorcon E
18. Dsstox_cid_25646
19. Dsstox_rid_81024
20. Dsstox_gsid_45646
21. Apexicon
22. 2-((6s,8s,9r,10s,11s,13s,14s,16s,17r)-17-acetoxy-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl Acetate
23. 6alpha-fluorobetamethasone-17,21 Diacetate
24. Einecs 251-575-1
25. Unii-7w2j09scwx
26. Brn 2318163
27. Pregna-1,4-diene-3,20-dione, 17,21-bis(acetyloxy)-6,9-difluoro-11-hydroxy-16-methyl-, (6.alpha.,11.beta.,16.beta.)-
28. Florone (tn)
29. Psorcon (tn)
30. Cas-33564-31-7
31. Diflorasone Diacetate [usan:usp:jan]
32. Acetic Acid Diflorasone
33. Opera_id_1660
34. Prestwick0_000619
35. Prestwick1_000619
36. Prestwick2_000619
37. Prestwick3_000619
38. Schembl4556
39. Bspbio_000558
40. Diflorasone 17,21-diacetate
41. Regid_for_cid_71414
42. Spbio_002777
43. Bpbio1_000614
44. Gtpl7068
45. Chembl1200545
46. Dtxsid8045646
47. Diflorasone Diacetate [mi]
48. Hms1569l20
49. Hms2096l20
50. Hms2231d10
51. Hms3713l20
52. Diflorasone Diacetate (jp17/usp)
53. Diflorasone Diacetate [jan]
54. 6.alpha.,9-difluoro-11.beta.,17,21-trihydroxy-16.beta.-methylpregna-1,4-diene-3,20-dione 17,21-diacetate
55. Zinc4212938
56. Diflorasone Diacetate [usan]
57. Tox21_110875
58. Diflorasone Diacetate [vandf]
59. Diflorasone Diacetate [mart.]
60. Akos025402039
61. Diflorasone Diacetate [usp-rs]
62. Diflorasone Diacetate [who-dd]
63. Tox21_110875_1
64. Ac-3515
65. Bcp9000608
66. Ccg-220619
67. Nsc 759267
68. Ncgc00022003-04
69. Ncgc00022003-06
70. (6alpha,11beta,16beta)-6,9-difluoro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl Diacetate
71. Pregna-1,4-diene-3,20-dione, 17,21-bis(acetyloxy)-6,9-difluoro-11-hydroxy-16-methyl-, (6alpha,11beta,16beta)-
72. Diflorasone Diacetate [orange Book]
73. Diflorasone Diacetate [usp Impurity]
74. Hy-107961
75. Ab00489907
76. Cs-0031025
77. Diflorasone Diacetate [usp Monograph]
78. D01327
79. E98631
80. Diflorasone Diacetate 100 Microg/ml In Methanol
81. 564d317
82. A821840
83. Sr-01000000122
84. Q-101374
85. Sr-01000000122-3
86. Brd-k17674993-001-03-1
87. Q27881705
88. Diflorasone Diacetate, United States Pharmacopeia (usp) Reference Standard
89. [2-[(6s,8s,9r,10s,11s,13s,14s,16s,17r)-17-acetyloxy-6,9-bis(fluoranyl)-10,13,16-trimethyl-11-oxidanyl-3-oxidanylidene-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxidanylidene-ethyl] Ethanoate
90. 6alpha,9-difluoro-11beta-hydroxy-16beta-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl Diacetate
91. Acetic Acid [2-[(6s,8s,9r,10s,11s,13s,14s,16s,17r)-17-acetyloxy-6,9-difluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Ester
92. Pregna-1,4-diene-3,20-dione, 17,21-bis(acetyloxy)-6,9-difluoro-11-hydroxy-16-methyl-, (6,alpha.,11.beta.,16.beta.)-
| Molecular Weight | 494.5 g/mol |
|---|---|
| Molecular Formula | C26H32F2O7 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 494.21160968 g/mol |
| Monoisotopic Mass | 494.21160968 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Diflorasone diacetate |
| Drug Label | Each gram of psorcon Ointment contains 0.5 mg diflorasone diacetate in an ointment base. Chemically, diflorasone diacetate is 6,9-difluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 17,21-diacetate. The structural formula is repre... |
| Active Ingredient | Diflorasone diacetate |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Fougera Pharms; Taro |
| 2 of 2 | |
|---|---|
| Drug Name | Diflorasone diacetate |
| Drug Label | Each gram of psorcon Ointment contains 0.5 mg diflorasone diacetate in an ointment base. Chemically, diflorasone diacetate is 6,9-difluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 17,21-diacetate. The structural formula is repre... |
| Active Ingredient | Diflorasone diacetate |
| Dosage Form | Ointment; Cream |
| Route | Topical |
| Strength | 0.05% |
| Market Status | Prescription |
| Company | Fougera Pharms; Taro |
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)


