



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
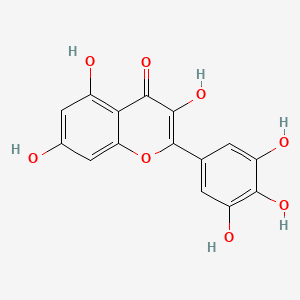

1. 3,3',4',5,5',7-hexahydroxyflavone
2. 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4h-1-benzopyran-4-one
3. Cannabiscetin
4. Delphidenolon 1575
1. 529-44-2
2. Cannabiscetin
3. Myricetol
4. 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4h-chromen-4-one
5. Myricitin
6. 3,3',4',5,5',7-hexahydroxyflavone
7. 3,5,7,3',4',5'-hexahydroxyflavone
8. 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one
9. 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4h-1-benzopyran-4-one
10. 3,3',4,4',5',7-hexahydro-2-phenyl-4h-chromen-4-one
11. 4h-1-benzopyran-4-one, 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-
12. Nsc 407290
13. Nsc-407290
14. Mfcd00006827
15. Nsc407290
16. Chembl164
17. 76xc01ftoj
18. Chebi:18152
19. Flavone, 3,3',4',5,5',7-hexahydroxy-
20. Myc
21. Smr001233193
22. Ccris 5838
23. Delphidenolon 1575
24. Sr-01000076005
25. Einecs 208-463-2
26. Unii-76xc01ftoj
27. Brn 0332331
28. Hsdb 7682
29. 4gqr
30. C15h10o8
31. Prestwick_342
32. Spectrum_001501
33. Specplus_000531
34. Myricetin [mi]
35. Myricetin [hsdb]
36. Myricetin [inci]
37. Prestwick0_000465
38. Prestwick1_000465
39. Prestwick2_000465
40. Prestwick3_000465
41. Spectrum4_001272
42. Spectrum5_000692
43. Lopac-m-6760
44. Myricetin (cannabiscetin)
45. Myricetin From Myrica Cerifera Leaf And Bark
46. Bidd:pxr0079
47. Lopac0_000740
48. Schembl19302
49. Bspbio_000570
50. Kbiogr_001884
51. Kbioss_001981
52. Mls002153825
53. Mls006010718
54. Bidd:er0142
55. Divk1c_006627
56. Myricetin, Analytical Standard
57. Spbio_002509
58. Bpbio1_000628
59. Megxp0_000357
60. Dtxsid8022400
61. Acon1_000267
62. Bdbm15236
63. Cid_5281672
64. Kbio1_001571
65. Kbio2_001981
66. Kbio2_004549
67. Kbio2_007117
68. 2o63
69. Chebi: 18152
70. Regid_for_cid_5281672
71. Hms1569m12
72. Hms2096m12
73. Hms2231l04
74. Hms3262c22
75. Hms3656i05
76. Myricetin - Cas 529-44-2
77. Bcp28295
78. Myricetin, >=96.0% (hplc)
79. Myricetin, >=96.0%, Crystalline
80. Tnp00286
81. Zinc3874317
82. Tox21_500740
83. Lmpk12110001
84. S2326
85. Stl284709
86. 3,7,3',4',5'-hexahydroxyflavone
87. Akos015903103
88. Ac-4533
89. Ccg-204825
90. Cs-6221
91. Db02375
92. Ks-5268
93. Lp00740
94. Sdccgsbi-0050718.p003
95. 3,3',4',5,5',7-hexoh-flavone
96. Flavone,3',4',5,5',7-hexahydroxy-
97. Ncgc00015697-01
98. Ncgc00015697-02
99. Ncgc00015697-03
100. Ncgc00015697-04
101. Ncgc00015697-05
102. Ncgc00015697-06
103. Ncgc00015697-07
104. Ncgc00015697-08
105. Ncgc00015697-09
106. Ncgc00015697-10
107. Ncgc00015697-11
108. Ncgc00015697-12
109. Ncgc00015697-13
110. Ncgc00015697-14
111. Ncgc00015697-25
112. Ncgc00094083-01
113. Ncgc00094083-02
114. Ncgc00094083-03
115. Ncgc00094083-04
116. Ncgc00179517-01
117. Ncgc00179517-02
118. Ncgc00261425-01
119. Cas-529-44-2
120. Hy-15097
121. Nci60_003870
122. Sy051702
123. Eu-0100740
124. Ft-0672573
125. M2131
126. N1850
127. Sw196616-2
128. M 6760
129. S00115
130. 3,3',4',5,5',7-hexahydroxy-(8ci)- Flavone
131. 529m442
132. A829320
133. Q951449
134. C07e0ed2-abf6-4bd3-a2b2-a98caef20fd1
135. Myricetin, Primary Pharmaceutical Reference Standard
136. Q-100601
137. Sr-01000076005-1
138. Sr-01000076005-6
139. Brd-k43149758-001-04-5
140. 3,3′,4′,5,5′,7-hexahydroxyflavone
141. Cannabiscetin; Hsdb 7682; Hsdb7682; Hsdb-7682
142. 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4h-chromen-4-one #
143. 4h-1-benzopyran-4-one,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-
| Molecular Weight | 318.23 g/mol |
|---|---|
| Molecular Formula | C15H10O8 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 1 |
| Exact Mass | 318.03756727 g/mol |
| Monoisotopic Mass | 318.03756727 g/mol |
| Topological Polar Surface Area | 148 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 506 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
... Significant quantities of quercetin and possibly myricetin and kaempferol are absorbed in the gut. A larger fraction probably remains in the lumen, and thus a substantial proportion of the gastrointestinal mucosa is exposed to biologically significant concentrations of these compounds. ...
PMID:11562264 Gee JM, Johnson IT; Curr Med Chem 8 (11): 1245-55 (2001)
Myricetin has known human metabolites that include (2S,3S,4S,5R)-6-[5,7-Dihydroxy-4-oxo-2-(3,4,5-trihydroxyphenyl)chromen-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Dietary polyphenols are a diverse and complex group of compounds that are linked to human health. Many of their effects have been attributed to the ability to poison (i.e., enhance DNA cleavage by) topoisomerase II. Polyphenols act against the enzyme by at least two different mechanisms. Some compounds are traditional, redox-independent topoisomerase II poisons, interacting with the enzyme in a noncovalent manner. Conversely, others enhance DNA cleavage in a redox-dependent manner that requires covalent adduction to topoisomerase II. Unfortunately, the structural elements that dictate the mechanism by which polyphenols poison topoisomerase II have not been identified. To resolve this issue, the activities of two classes of polyphenols against human topoisomerase IIalpha were examined. The first class was a catechin series, including (-)-epigallocatechin gallate (EGCG), (-)-epigallocatechin (EGC), (-)-epicatechin gallate (ECG), and (-)-epicatechin (EC). The second was a flavonol series, including myricetin, quercetin, and kaempferol. Compounds were categorized into four distinct groups: EGCG and EGC were redox-dependent topoisomerase II poisons, kaempferol and quercetin were traditional poisons, myricetin utilized both mechanisms, and ECG and EC displayed no significant activity. On the basis of these findings, a set of rules is proposed that predicts the mechanism of bioflavonoid action against topoisomerase II. The first rule centers on the B ring. While the C4'-OH is critical for the compound to act as a traditional poison, the addition of -OH groups at C3' and C5' increases the redox activity of the B ring and allows the compound to act as a redox-dependent poison. The second rule centers on the C ring. The structure of the C ring in the flavonols is aromatic and planar and includes a C4-keto group that allows the formation of a proposed pseudo ring with the C5-OH. Disruption of these elements abrogates enzyme binding and precludes the ability to function as a traditional topoisomerase II poison.
PMID:18461976 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2737509 Bandele OJ et al; Chem Res Toxicol 21 (6): 1253-60 (2008)
Selected flavonoids were tested for their ability to inhibit the catalytic activity of DNA topoisomerase (topo) I and II. Myricetin, quercetin, fisetin, and morin were found to inhibit both enzymes, while phloretin, kaempferol, and 4',6,7-trihydroxyisoflavone inhibited topo II without inhibiting topo I. Flavonoids demonstrating potent topo I and II inhibition required hydroxyl group substitution at the C-3, C-7, C-3', and C-4' positions and also required a keto group at C-4. Additional B-ring hydroxylation enhanced flavonoid topo I inhibitory action. A C-2, C-3 double bond was also required, but when the A ring is opened, the requirement for the double bond was eliminated. Genistein has been previously reported to stabilize the covalent topo II-DNA cleavage complex and thus function as a topo II poison. All flavonoids were tested for their ability to stabilize the cleavage complex between topo I or topo II and DNA. None of the agents stabilized the topo I-DNA cleavage complex, but prunetin, quercetin, kaempferol, and apigenin stabilized the topo II DNA-complex. Competition experiments have shown that genistein-induced topo II-mediated DNA cleavage can be inhibited by myricetin, suggesting that both types of inhibitors (antagonists and poisons) interact with the same functional domain of their target enzyme...
PMID:7769390 Constantinou A et al; J Nat Prod 58 (2): 217-25 (1995)
... myricetin (3, 3', 4', 5, 5', 7-hexahydroxyflavone) ... could directly bind to JAK1/STAT3 molecules to inhibit cell transformation in epidermal growth factor (EGF)-activated mouse JB6 P(+) cells. Colony assay revealed that myricetin had the strongest inhibitory effect on cell transformation among three flavonols including myricetin, quercetin and kaempferol. Molecular data revealed that myricetin inhibited DNA- binding and transcriptional activity of STAT3. Furthermore, myricetin inhibited the phosphorylation of STAT3 at Tyr705 and Ser727. Cellular signaling analyses revealed that EGF could induce the phosphorylation of Janus Kinase (JAK) 1, but not JAK2. Myricetin inhibited the phosphorylation of JAK1 and increased the autophosphorylation of EGF receptor (EGFR). Moreover, ex vivo and in vitro pull-down assay revealed that myricetin bound to JAK1 and STAT3, but not EGFR. Affinity data further demonstrated that myricetin had a higher affinity for JAK1 than STAT3. Thus, ... myricetin might directly target JAK1 to block cell transformation in mouse JB6 cells.
PMID:18995957 Kumamoto T et al; Cancer Lett. 2008 Nov 6. (Epub ahead of print)
Abnormal expression of cyclooxygenase-2 (COX-2) has been implicated in the development of cancer. ... Here /it is reported/ that 3,3',4',5,5',7-hexahydroxyflavone (myricetin), one of the major flavonols in red wine, inhibits 12-O-tetradecanoylphorbol-13-acetate (phorbol ester)-induced COX-2 expression in JB6 P+ mouse epidermal (JB6 P+) cells by suppressing activation of nuclear factor kappa B (NF-kappaB). Myricetin at 10 and 20 uM inhibited phorbol ester-induced upregulation of COX-2 protein, while resveratrol at the same concentration did not exert significant effects. The phorbol ester-induced production of prostaglandin E 2 was also attenuated by myricetin treatment. Myricetin inhibited both COX-2 and NF-kappaB transactivation in phorbol ester-treated JB6 P+ cells, as determined using a luciferase assay. Myricetin blocked the phorbol ester-stimulated DNA binding activity of NF-kappaB, as determined using an electrophoretic mobility shift assay. Moreover, TPCK (N-tosyl-l-phenylalanine chloromethyl ketone), a NF-kappaB inhibitor, significantly attenuated COX-2 expression and NF-kappaB promoter activity in phorbol ester-treated JB6 P+ cells. In addition, red wine extract inhibited phorbol ester-induced COX-2 expression and NF-kappaB transactivation in JB6 P+ cells. Collectively, these data suggest that myricetin contributes to the chemopreventive effects of red wine through inhibition of COX-2 expression by blocking the activation of NF-kappaB.
PMID:17944529 Lee KM et al; J Agric Food Chem 55 (23): 9678-84 (2007)
For more Mechanism of Action (Complete) data for MYRICETIN (6 total), please visit the HSDB record page.


