



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
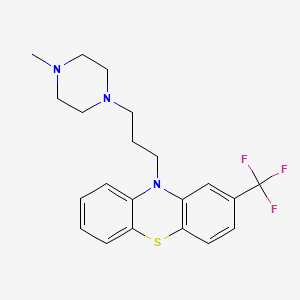

1. Apo Trifluoperazine
2. Apo-trifluoperazine
3. Apotrifluoperazine
4. Eskazine
5. Flupazine
6. Stelazine
7. Terfluzine
8. Trifluoperazine Hcl
9. Trifluoperazine Hydrochloride
10. Trifluoroperazine
11. Trifluperazine
12. Triftazin
1. Trifluperazine
2. 117-89-5
3. Trifluoroperazine
4. Flurazine
5. Trifluoperazin
6. Triflurin
7. Triperazine
8. Trifluoperazina
9. Trifluoromethylperazine
10. Trifluoperazinum
11. Trifluroperizine
12. Apo-trifluoperazine
13. Eskazine
14. Rp 7623
15. Fluoperazine
16. 10-[3-(4-methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)phenothiazine
17. Trifluoromethyl-10-(3'-(1-methyl-4-piperazinyl)propyl)phenothiazine
18. 10-[3-(4-methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)-10h-phenothiazine
19. Tfp
20. Nsc 17474
21. 10-(3-(4-methylpiperazin-1-yl)propyl)-2-(trifluoromethyl)-10h-phenothiazine
22. 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-10h-phenothiazine
23. Calmazine
24. Trifluoperazine (inn)
25. 10-(3-(4-methyl-1-piperazinyl)propyl)-2-(trifluoromethyl)phenothiazine
26. 10h-phenothiazine, 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-
27. Nsc-17474
28. 10-(gamma-(n'-methylpiperazino)propyl)-2-trifluoromethylphenothiazine
29. 10h-phenothiazine, 10-(3-(4-methyl-1-piperazinyl)propyl)-2-(trifluoromethyl)-
30. 10-[3-(4-methyl-piperazin-1-yl)-propyl]-2-trifluoromethyl-10h-phenothiazine
31. Skf 5019
32. Chebi:45951
33. 214izi85k3
34. 2-trifluoromethyl-10-(3'-(1-methyl-4-piperazinyl)propyl)phenothiazine
35. Phenothiazine, 10-(3-(4-methyl-1-piperazinyl)propyl)-2-(trifluoromethyl)-
36. Nsc-46061
37. Rp-7623
38. Nsc17474
39. Trifluoperazina [italian]
40. Trifluoperazine [inn]
41. Trifluoperazine [inn:ban]
42. Trifluoperazinum [inn-latin]
43. Trifluoperazina [inn-spanish]
44. Mls001146870
45. Mls002702821
46. 2-trifluoromethyl-10-[3'-(1-methyl-4-piperazinyl)propyl]phenothiazine
47. 10-(3-(4-methyl-1-piperazinyl)propyl)-2-(trifluoromethyl)-10h-phenothiazine
48. Smr001566649
49. Ccris 6994
50. Hsdb 3195
51. 10-[3-(4-methylpiperazin-1-yl)propyl]-2-(trifluoromethyl)phenothiazine Dihydrochloride
52. Cas-440-17-5
53. Einecs 204-219-4
54. Apo-trifluoperazine (tn)
55. Nsc 46061
56. Stelazine (*dihydrochloride*)
57. Unii-214izi85k3
58. Phenothiazine, 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-
59. Nci17474
60. Synklor (salt/mix)
61. Stelazine (salt/mix)
62. Triftazin (salt/mix)
63. Spectrum_000668
64. Terfluzine (salt/mix)
65. Triftazine (salt/mix)
66. Jatroneural (salt/mix)
67. Fluoperazine (salt/mix)
68. Triphthazine (salt/mix)
69. Prestwick0_000313
70. Prestwick1_000313
71. Prestwick2_000313
72. Prestwick3_000313
73. Spectrum2_000828
74. Spectrum3_001374
75. Spectrum4_000368
76. Spectrum5_001553
77. Lopac-t-8516
78. Biomol-nt_000060
79. Chembl422
80. Cid_5566
81. 2-(((4-chlorophenyl)sulfonyl)amino)-benzoicaci
82. Ncistruc1_001127
83. Ncistruc2_001093
84. Trifluoperazine [mi]
85. Bidd:pxr0132
86. Lopac0_001232
87. Schembl24866
88. Bspbio_000306
89. Bspbio_001190
90. Bspbio_002928
91. Gtpl214
92. Kbiogr_000530
93. Kbiogr_000835
94. Kbiogr_002431
95. Kbioss_000530
96. Kbioss_001148
97. Kbioss_002437
98. Mls006011857
99. Divk1c_000843
100. Trifluoperazine [hsdb]
101. Spbio_000755
102. Spbio_002525
103. Trifluoperazine [vandf]
104. Bpbio1_000338
105. Bpbio1_001345
106. Dtxsid1046928
107. Trifluoperazine [who-dd]
108. Bdbm79181
109. Cid_2913535
110. Kbio1_000843
111. Kbio2_000530
112. Kbio2_001148
113. Kbio2_002431
114. Kbio2_003098
115. Kbio2_003716
116. Kbio2_004999
117. Kbio2_005666
118. Kbio2_006284
119. Kbio2_007567
120. Kbio3_000959
121. Kbio3_000960
122. Kbio3_002148
123. Kbio3_002910
124. Cmap_000048
125. Ninds_000843
126. Bio1_000458
127. Bio1_000947
128. Bio1_001436
129. Bio2_000435
130. Bio2_000915
131. Hms1362l11
132. Hms1792l11
133. Hms1990l11
134. Hms2089j11
135. Hms3429o07
136. Kuc109776n
137. Bcp32898
138. Ex-a3330
139. Ccg-37306
140. Ncgc00013226
141. Pdsp1_001300
142. Pdsp2_001284
143. S5856
144. Stk182873
145. Zinc19418959
146. 10-(3-(4-methyl-1-piperazinyl)propyl)-2-(trifluoromethyl)-phenothiazine
147. Akos001487920
148. Db00831
149. Sdccgsbi-0051199.p005
150. Idi1_000843
151. Idi1_002190
152. Ksc-210-031
153. Mrf-0000088
154. Qtl1_000085
155. Ncgc00013226-02
156. Ncgc00013226-03
157. Ncgc00013226-04
158. Ncgc00013226-05
159. Ncgc00013226-06
160. Ncgc00013226-07
161. Ncgc00013226-08
162. Ncgc00013226-09
163. Ncgc00013226-10
164. Ncgc00013226-11
165. Ncgc00013226-12
166. Ncgc00013226-13
167. Ncgc00013226-15
168. Ncgc00013226-26
169. Ncgc00024251-03
170. Ncgc00024251-04
171. Ncgc00024251-05
172. Ncgc00024251-06
173. Ncgc00024251-07
174. Ac-35814
175. Nci60_001427
176. Nci60_004087
177. Sbi-0051199.p003
178. Ab00053558
179. Ft-0650159
180. C07168
181. D08636
182. Ab00053558-27
183. Ab00053558_28
184. Ab00053558_29
185. L001075
186. Q1752915
187. Sr-01000003020-6
188. Brd-k89732114-001-02-6
189. Brd-k89732114-001-03-4
190. Brd-k89732114-001-05-9
191. Brd-k89732114-300-05-5
192. Brd-k89732114-300-07-1
193. Triphthazine;trifluperazine;nsc-17474;rp-7623;skf-5019
194. 10-(.gamma.-(n'-methylpiperazino)propyl)-2-trifluoromethylphenothiozine
195. 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-10h-phenothiazine #
196. 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)phenothiazine;hydrochloride
197. 10-[3-(4-methylpiperazino)propyl]-2-(trifluoromethyl)phenothiazine;hydrochloride
| Molecular Weight | 407.5 g/mol |
|---|---|
| Molecular Formula | C21H24F3N3S |
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 4 |
| Exact Mass | 407.16430344 g/mol |
| Monoisotopic Mass | 407.16430344 g/mol |
| Topological Polar Surface Area | 35 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 510 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiemetics; Antipsychotic Agents, Phenothiazine; Dopamine Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
IN THE TREATMENT OF ACUTE PSYCHOSES, THE DOSE OF ANTIPSYCHOTIC DRUG IS INCREASED DURING THE FIRST FEW DAYS TO ACHIEVE CONTROL OF SYMPTOMS. THE DOSE IS THEN ADJUSTED DURING THE NEXT SEVERAL WEEKS AS THE PATIENT'S CONDITION WARRANTS. /PHENOTHIAZINES/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 418
The use of antipsychotic drugs in mania and depression has met with some success. /PHENOTHIAZINES/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 419
...TRIFLUOPERAZINE...REPORTED EFFECTIVE IN SEVERELY IMPAIRED AUTISTIC CHILDREN, & DIFFERENTIAL DIAGNOSIS OF THIS GROUP FROM SO-CALLED MINIMAL-BRAIN-DAMAGE (MBD) SYNDROME IN CHILDREN IS ESSENTIAL.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 174
For more Therapeutic Uses (Complete) data for TRIFLUOPERAZINE (10 total), please visit the HSDB record page.
PHENOTHIAZINES SHOULD BE USED WITH EXTREME CAUTION, IF @ ALL, IN UNTREATED EPILEPTIC PT & IN PT UNDERGOING WITHDRAWAL FROM CENTRAL DEPRESSANT DRUGS SUCH AS ALCOHOL & BARBITURATES. /PHENOTHIAZINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 159
...FEW PT WITH ANGINA PECTORIS HAVE REPORTED INCR PAIN DURING THERAPY WITH TRIFLUOPERAZINE. THEREFORE, PT WITH ANGINA SHOULD BE OBSERVED CAREFULLY & DRUG WITHDRAWN IF UNFAVORABLE RESPONSE OCCURS. /HYDROCHLORIDE/
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 440
SAFE USE...DURING PREGNANCY HAS NOT BEEN ESTABLISHED WITH RESPECT TO POSSIBLE ADVERSE EFFECTS ON FETAL DEVELOPMENT. ...PHENOTHIAZINES... SHOULD BE USED WITH EXTREME CAUTION IN PT WITH HISTORY OF GLAUCOMA OR PROSTATIC HYPERTROPHY. /PHENOTHIAZINES/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1021
ROUTINE ADMIN OF ANTIPARKINSONISM AGENTS SHOULD BE AVOIDED WITH MOST ANTIPSYCHOTIC DRUG REGIMENS. /PHENOTHIAZINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 172
For more Drug Warnings (Complete) data for TRIFLUOPERAZINE (28 total), please visit the HSDB record page.
Lethal dose: 0.3-0.8 mg/dL /From table/
Gossel, T.A., J.D. Bricker. Principles of Clinical Toxicology. 3rd ed. New York, NY: Raven Press, Ltd., 1994., p. 422
For the treatment of anxiety disorders, depressive symptoms secondary to anxiety and agitation.
Trifluoperazine is a trifluoro-methyl phenothiazine derivative intended for the management of schizophrenia and other psychotic disorders. Trifluoperazine has not been shown effective in the management of behaviorial complications in patients with mental retardation.
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AB - Phenothiazines with piperazine structure
N05AB06 - Trifluoperazine
ULTIMATE SOJOURN OF PHENOTHIAZINE DRUGS IN BODY IS EXCEEDINGLY LONG. /PHENOTHIAZINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 163
Hepatic.
AFTER CHRONIC ADMIN OF PIPERAZINE-SUBSTITUTED PHENOTHIAZINE DRUGS...TO RATS, TISSUES CONTAINED DRUG METABOLITES, IN WHICH PIPERAZINE RING FISSION BY MULTIPLE OXIDATIVE N-DEALKYLATION HAD OCCURRED TO GIVE SUBSTITUTED ETHYLENEDIAMINE. ...2-TRIFLUOROMETHYL ANALOGUE WAS FORMED SIMILARLY FROM TRIFLUPERAZINE.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 255
IN VIVO DEGRADATION OF PIPERAZINE RING OF TRIFLUOPERAZINE LEADS TO FORMATION OF GAMMA-(PHENOTHIAZINYL-10)-PROPYLAMINE & ITS RING SUBSTITUTED ANALOGS CF3- & CL-. SULFOXIDES OF THESE METABOLITES HAVE BEEN IDENTIFIED AS URINARY BIOTRANSFORMATION PRODUCTS IN RATS (CHRONIC).
PMID:4813348 BREYER ET AL; BIOCHEM PHARM 23: 313-322 (1974)
10-20 hours
Trifluoperazine blocks postsynaptic mesolimbic dopaminergic D1 and D2 receptors in the brain; depresses the release of hypothalamic and hypophyseal hormones and is believed to depress the reticular activating system thus affecting basal metabolism, body temperature, wakefulness, vasomotor tone, and emesis.
...PHENOTHIAZINES, BLOCK DOPAMINE RECEPTORS & INCR TURNOVER RATE OF DOPAMINE IN CORPUS STRIATUM. INCR TURNOVER RATE IS BELIEVED TO BE RESULT OF NEURONAL FEEDBACK MECHANISM. ...OF IDENTIFIED DOPAMINERGIC NEURONS IN SUBSTANTIA NIGRA & VENTRAL TEGMENTAL AREAS. SPONTANEOUS FIRING OF THESE CELLS IS INCR... /PHENOTHIAZINES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 159


