



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
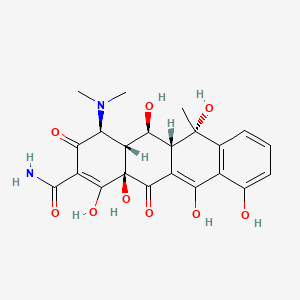

1. Bisolvomycin
2. Geomycin
3. Hydroxytetracycline
4. Oxyterracin
5. Oxyterracine
6. Oxytetracid
7. Oxytetracycline Anhydrous
8. Oxytetracycline Calcium
9. Oxytetracycline Dihydrate
10. Oxytetracycline Hydrochloride
11. Oxytetracycline Monohydrochloride
12. Oxytetracycline Sulfate (2:1)
13. Oxytetracycline, (4a Beta,5 Beta,5a Beta,12a Beta)-isomer
14. Oxytetracycline, (5 Beta)-isomer
15. Oxytetracycline, Anhydrous
16. Oxytetracycline, Calcium (1:1) Salt
17. Oxytetracycline, Disodium Salt, Dihydrate
18. Oxytetracycline, Sodium Salt
19. Terramycin
1. 79-57-2
2. Terramycin
3. Oxyterracine
4. Oxytetracyclin
5. Adamycin
6. Oxymycin
7. Terrafungine
8. Oxyterracin
9. Oxytetracycline Anhydrous
10. Oxitetraciclina
11. Oxitetracyclin
12. Biostat
13. Geomycin
14. Ryomycin
15. Oxytetracycline (anhydrous)
16. Oxitetracyclinum
17. Oxytetracyclinum
18. Oxyterracyne
19. Solkaciclina
20. Dabicycline
21. Fanterrin
22. Geotilin
23. Imperacin
24. Lenocycline
25. Oksisyklin
26. Proteroxyna
27. Riomitsin
28. Terramitsin
29. Ursocyclin
30. Ursocycline
31. Oxypam
32. Tarocyn
33. Tarosin
34. Teravit
35. Tetran
36. Antibiotic Tm 25
37. Oxytetracycline Amphoteric
38. 5-hydroxytetracycline
39. Liquamycin La 200
40. Biostat Pa
41. Oxytetracycline [inn]
42. Bisolvomycin
43. Oxysteclin
44. Oxymykoin
45. Vendarcin
46. Ossitetraciclina
47. Pennox 200
48. Oxy-kesso-tetra
49. Oxytetracycline (terramycin)
50. Berkmycen
51. Mycoshield Tmqthc 20
52. Dalimycin
53. Oxacycline
54. Unimycin
55. Nsc-9169
56. Oxytetracycline Calcium
57. Stevacin
58. Macocyn
59. Medamycin
60. Mepatar
61. (4s,4ar,5s,5ar,6s,12as)-4-(dimethylamino)-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
62. Otc (antibiotic)
63. Mls000069429
64. Nsc 9169
65. Slf0d9077s
66. Tetracycline, 5-hydroxy-
67. Chebi:27701
68. Oxitetracycline
69. Terramycine
70. Nitox
71. Oxytetracycline (internal Use)
72. Stecsolin
73. Oxydon
74. Tm 5
75. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-
76. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-, (4s,4ar,5s,5ar,6s,12as)-
77. Oxytetracid
78. E703
79. Nci-c56473
80. Smr000059000
81. Terramycin Im
82. Terramycin Q50
83. Ossitetraciclina [dcit]
84. (4s,4ar,5s,5ar,6s,12as)-4-(dimethylamino)-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide
85. Oxytetracycline Base
86. Oxytetracyclinum [inn-latin]
87. Oxitetraciclina [inn-spanish]
88. Terramicina Oftalmica
89. Geomycin (streptomyces Vimosus)
90. Hsdb 3145
91. Nsc-757262
92. Oxytetracycline Dehydrate
93. Einecs 201-212-8
94. La 200
95. Brn 2714587
96. Unii-slf0d9077s
97. Embryostat
98. Galsenomycin
99. Sr-01000003006
100. Hydroxytetracyclinum
101. (4s,4ar,5s,5ar,6s,12ar)-4-(dimethylamino)-1,5,6,10,11,12a-hexahydroxy-6-methyl-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide
102. Oxytetracycline,(s)
103. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-, (4s-(4alpha,4aalpha,5alpha,5aalpha,6beta,12aalpha))-
104. 5-hydroxy-tetracycline
105. Spectrum_001055
106. Opera_id_661
107. Oxytetracyclinum Dihydras
108. Prestwick0_000307
109. Prestwick1_000307
110. Prestwick2_000307
111. Prestwick3_000307
112. Spectrum2_000988
113. Spectrum3_000536
114. Spectrum4_000466
115. Spectrum5_001148
116. Oxytetracycline(terramycin)
117. Schembl2899
118. Chembl1517
119. Dsstox_cid_14260
120. Dsstox_rid_79133
121. Oxytetracycline [mi]
122. Dsstox_gsid_34260
123. Bspbio_000274
124. Bspbio_002151
125. Doxycycline Impurity E
126. Kbiogr_000912
127. Kbioss_001535
128. 4-14-00-02633 (beilstein Handbook Reference)
129. Divk1c_000225
130. Schembl560497
131. Spbio_001055
132. Spbio_002493
133. Bpbio1_000302
134. Chembl461529
135. Chembl1401333
136. Chembl4280957
137. Nsc9169 (hcl)
138. Oxytetracycline [who-dd]
139. Schembl13169109
140. Schembl13782651
141. Gtpl10919
142. Kbio1_000225
143. Kbio2_001535
144. Kbio2_004103
145. Kbio2_006671
146. Kbio3_001651
147. 6153-64-6 (di-hydrate)
148. Ninds_000225
149. Bdbm241973
150. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-, (4s-(4.alpha.,4a.alpha.,5.alpha.,5a.alpha.,6.beta.,12a.alpha.))-
151. 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide
152. 79-57-2 (anhydrous)
153. Hy-b0275
154. 2058-46-0 (mono-hydrochloride)
155. Tox21_302380
156. Lmpk07000005
157. Mfcd00003700
158. S1773
159. Zinc95626782
160. Akos015951277
161. Akos015961254
162. Zinc100026355
163. Zinc100303028
164. Ccg-269334
165. Db00595
166. Cas-79-57-2
167. Idi1_000225
168. Ncgc00091268-04
169. Ncgc00091268-05
170. Ncgc00091268-06
171. Ncgc00091268-07
172. Ncgc00091268-08
173. Ncgc00091268-09
174. Ncgc00091268-10
175. Ncgc00091268-11
176. Ncgc00091268-12
177. Ncgc00188956-01
178. Ncgc00255168-01
179. Ac-12777
180. Ac-13466
181. 7179-50-2 (calcium (1:1) Salt)
182. Oxytetracycline (anhydrous) [hsdb]
183. Sbi-0051473.p003
184. Sw196796-3
185. 3,5,6,10,12,12a-hexahydroxy-6-methyl-
186. C06624
187. E75911
188. Oxytetra Selective Supplement, For Microbiology
189. Oxytetracycline 1000 Microg/ml In Acetonitrile
190. Ab00053514_04
191. Ab00053514_05
192. Ab01274728-01
193. Ab01274728_02
194. 6153-65-7 (di-hydrochloride Salt, Di-hydrate)
195. A839720
196. Q411646
197. (4s,4ar,5s,5ar,6s,12as)-4-(dimethylamino)-
198. Doxycycline Hyclate Impurity E [ep Impurity]
199. Sr-01000003006-5
200. Q63393012
201. Doxycycline Monohydrate Impurity E [ep Impurity]
202. 1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene
203. Oxytetracycline, British Pharmacopoeia (bp) Reference Standard
204. Oxytetracycline, European Pharmacopoeia (ep) Reference Standard
205. (4s,4ar,5s,5ar,6s,12as)-4-(dimethylamino)-3,5,6,10,12,12ahexahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
206. 2z,4s,4ar,5s,5ar,6s,12as)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,6,10,11,12a-pentahydroxy-6-methyl-4,4a,5,5a-tetrahydrotetracene-1,3,12-trione
| Molecular Weight | 460.4 g/mol |
|---|---|
| Molecular Formula | C22H24N2O9 |
| XLogP3 | -1.6 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 2 |
| Exact Mass | 460.14818035 g/mol |
| Monoisotopic Mass | 460.14818035 g/mol |
| Topological Polar Surface Area | 202 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 1000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Mesh Heading: anti-bacterial agents
National Library of Medicine, SIS; ChemIDplus Record for Oxytetracycline (79-57-2). Available from, as of April 13, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Antibiotics, Tetracycline
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
... Possess wide range of antimicrobial activity against gram-positive and gram-negative bacteria ... some microorganisms innately insensitive to many chemotherapeutic agents, such as rickettsiae, mycoplasma, chlamydia agents of lymphogranuloma venerum, psittacosis, inclusion conjunctivitis, and trachoma and amebae. /Tetracyclines/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1117
The tetracyclines are active against a wide range of aerobic and anaerobic gram-positive and gram-negative bacteria. They also are effective against some microorganisms that are resistant to cell-wall-active antimicrobial agents, such as Rickettsiae, Coxiella burnetii, Mycoplasma pneumoniae, Chlamydia spp, Legionella spp, Ureaplasma, some atypical mycobacteria, and Plasmodium spp. They are not active against fungi. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1240
For more Therapeutic Uses (Complete) data for OXYTETRACYCLINE (28 total), please visit the HSDB record page.
GENERIC INEQUIVALENCE HAS BEEN DEMONSTRATED FOR SOME OXYTETRACYCLINE FORMULATIONS, ALTHOUGH INDIVIDUAL VARIATION PREVENTED SIGNIFICANT DIFFERENCES BEING SHOWN IN ALL BUT MOST EXTREME CASES.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 172
FOOD, MILK, NONSYSTEMIC ANTACIDS & IRON PREPN INTERFERE WITH ORAL ABSORPTION.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1142
... Not active against any true viruses, yeasts, or fungi. /Tetracyclines/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1184
Topical admin is best avoided because of high risk of sensitization, except for use in eye ... Should never be injected intrathecally. /Tetracyclines/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1120
For more Drug Warnings (Complete) data for OXYTETRACYCLINE (38 total), please visit the HSDB record page.
Oxytetracycline is indicated for treatment of infections caused by a variety of Gram positive and Gram negative microorganisms including Mycoplasma pneumoniae, Pasteurella pestis, Escherichia coli, Haemophilus influenzae (respiratory infections), and Diplococcus pneumoniae.
Oxytetracycline is known as a broad-spectrum antibiotic due to its activity against such a wide range of infections. It was the second of the tetracyclines to be discovered. Oxytetracycline, like other tetracyclines, is used to treat many infections common and rare. Its better absorption profile makes it preferable to tetracycline for moderately severe acne, but alternatives sould be sought if no improvement occurs by 3 months.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AA - Tetracycline and derivatives
D06AA03 - Oxytetracycline
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AA - Antibiotics
G01AA07 - Oxytetracycline
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01A - Tetracyclines
J01AA - Tetracyclines
J01AA06 - Oxytetracycline
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AA - Antibiotics
S01AA04 - Oxytetracycline
Absorption
Readily absorbed following oral administration.
SERUM HALF-LIFE ... IN HORSES IS ... 15.7 HR & 10.5 HR AFTER IV & IM INJECTIONS, RESPECTIVELY. ... /A FACTOR/ MAY BE THE INFLUENCE OF DOSE-DEPENDENT KINETICS ... .
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 174
The percentage of an oral dose that is absorbed (when the stomach is empty) ... for oxytetracycline /is/ 60 to 80% ... After a single oral dose, the peak plasma concn /of oxytetracycline/ is attained in 2 to 4 hr. /It has a half-life/ in the range of 6 to 12 hr and ... frequently admin 2 to 4 times daily ... The admin of 250 mg every 6 hr produces peak plasma concn of 2 to 2.5 ug/mL ... Increasing the dosage above 1 g every 6 hr does not produce significantly higher plasma concn ... Approx 10 to 35% of a dose of oxytetracycline is excreted in active form in urine, in which it is detectable within 30 min and reaches a peak concn about 5 hr after it is admin.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1242
/Oxytetracycline is/ bound to plasma proteins ... approx ... 20-25%.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1119
/Absorption is/ much less complete from lower ... tract ... Biliary concn ... /is/ 5 to 10 times higher than ... plasma. /Tetracyclines/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1119
For more Absorption, Distribution and Excretion (Complete) data for OXYTETRACYCLINE (20 total), please visit the HSDB record page.
BIOLOGIC HALF-LIFE ... MAY BE 3-4 DAYS IN ANURIA.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1142
The serum half-life of oxytetracycline is 6 to 10 hours in adults with normal renal function and is reported to be 47 to 66 hours in patients with severe renal impairment. In patients with normal renal function, approximately 60 to 70 percent of a single oral dose of oxytetracycline is excreted in urine within 72 hours as active drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 319
A two-way crossover study was conducted in crossbred male calves (6-8 months old) to determine the bioavailability, pharmacokinetics and dosage regimens for a long-acting formulation of oxytetracycline (OTC-LA). The half-lives of oxytetracycline after intravenous and intramuscular administration were 7.8 hr and 24 hr, respectively. ....
PMID:10066127 Kumar R, Malik JK; Vet Res Commun 22 (8): 533-44 (1998)
The pharmacokinetic properties of oxytetracycline were studied following a single injection of a long-acting formulation (20 mg/kg body weight) into the semimembranosus muscle of healthy dogs and of dogs that had been experimentally infected with Ehrlichia canis. ... The mean apparent elimination half-life (t(1/2) beta) was significantly increased following infection. ... The absorption half-life (t(1/2) ab) was significantly decreased after infection.
PMID:11469510 Kikuvi GM et al; Vet Res Commun 25 (5): 391-400 (2001)
SERUM HALF-LIFE ... IN HORSES IS ... 15.7 HR & 10.5 HR AFTER IV & IM INJECTIONS, RESPECTIVELY. ... /A FACTOR/ MAY BE THE INFLUENCE OF DOSE-DEPENDENT KINETICS ... .
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 174
Oxytetracycline inhibits cell growth by inhibiting translation. It binds to the 30S ribosomal subunit and prevents the amino-acyl tRNA from binding to the A site of the ribosome. The binding is reversible in nature. Oxytetracycline is lipophilic and can easily pass through the cell membrane or passively diffuses through porin channels in the bacterial membrane.
Tetracyclines inhibit bacterial protein synthesis by binding to the 30 S bacterial ribosome and preventing access of aminoacyl tRNA to the acceptor (A) site on the mRNA-ribosome complex. They enter gram-negative bacteria by passive diffusion through the hydrophilic channels formed by the porin proteins of the outer cell membrane, and active transport by an energy-dependent system that pumps all tetracyclines across cytoplasmic membrane. Although permeation of these drugs into gram-positive bacteria is less well understood, it also is energy requiring. At high concn, these cmpd impair protein synthesis in mammalian cells. However, because mammalian cells lack the active transport system found in bacteria, and the ribosomal target is less sensitive, tetracyclines are selectively active against bacteria. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1241
The tetracycline antibiotics ... can produce neuromuscular blockade, possibly by chelation of Ca+2. /Tetracyclines/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 203






