



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
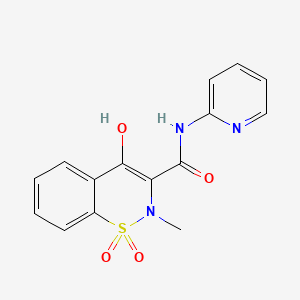

1. Cp 16171
2. Cp-16171
3. Cp16171
4. Feldene
1. 36322-90-4
2. Feldene
3. Piroxicamum
4. Pyroxycam
5. Roxicam
6. Piroftal
7. Baxo
8. Cp-16171
9. 2h-1,2-benzothiazine-3-carboxamide, 4-hydroxy-2-methyl-n-2-pyridinyl-, 1,1-dioxide
10. 4-hydroxy-2-methyl-n-(pyridin-2-yl)-2h-benzo[e][1,2]thiazine-3-carboxamide 1,1-dioxide
11. Cp 16171
12. Chf 1251
13. Nsc 666076
14. Piroxicam (feldene)
15. 4-hydroxy-2-methyl-n-(2-pyridyl)-2h-1,2-benzothiazine-3-carboxamide-1,1-dioxide
16. Nsc-666076
17. 13t4o6vmam
18. 4-hydroxy-2-methyl-3-(pyrid-2-yl-carbamoyl)-2h-1,2-benzothiazine 1,1-dioxide
19. 4-hydroxy-2-methyl-n-(2-pyridyl)-2h-1,2-benzothiazin-3-caboxyamid-1,1-dioxid
20. 4-hydroxy-2-methyl-n-2-pyridyl-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
21. Cp-16,171
22. Mls000069644
23. Chebi:8249
24. Nsc666076
25. 4-hydroxy-2-methyl-n-pyridin-2-yl-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
26. Ncgc00015823-02
27. Artroxicam
28. Bruxicam
29. Caliment
30. Flogobene
31. Geldene
32. Improntal
33. Larapam
34. Piroflex
35. Reudene
36. Roxiden
37. Sasulen
38. Smr000035997
39. Solocalm
40. Erazon
41. Pirkam
42. Piroxicam Usp
43. Riacen
44. Zunden
45. Roxam
46. Cas-36322-90-4
47. Dsstox_cid_1170
48. 4-hydroxy-2-methyl-1,1-dioxo-n-pyridin-2-yl-1lambda6,2-benzothiazine-3-carboxamide
49. 4-hydroxy-2-methyl-n-2-pyridinyl-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
50. Dsstox_rid_75990
51. Dsstox_gsid_21170
52. Piroxicamum [inn-latin]
53. Piroxicam D3 (n-methyl D3)
54. Rosiden
55. Felden
56. Feldene Fast
57. Feldene Gel
58. 4-hydroxy-2-methyl-n-(2-pyridyl)-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
59. 4-hydroxy-2-methyl-n-2-pyridinyl-2h-1,2-benzothiazine-3-carboxamide-1,1-dioxide
60. (z)-3-(hydroxy(pyridin-2-ylamino)methylene)-2-methyl-2h-benzo[e][1,2]thiazin-4(3h)-one 1,1-dioxide
61. Feldene (tn)
62. Ccris 3719
63. Sr-01000000199
64. Einecs 252-974-3
65. Ak1015
66. Unii-13t4o6vmam
67. Brn 0627692
68. 4-hydroxy-2-methyl-1,1-dioxo-n-pyridin-2-yl-1?^{6},2-benzothiazine-3-carboxamide
69. Piroxicam,(s)
70. Prestwick_573
71. Mfcd00057317
72. 4-hydroxy-2-methyl-n-(2-pyridyl)-2h-1,2-benzothiazin-3-caboxyamid-1,1-dioxid [german]
73. Piroxicam [usan:usp:inn:ban:jan]
74. Piroxicam:malonic Acid
75. Spectrum_001115
76. Tocris-0960
77. Piroxicam-(methyl-d3)
78. Opera_id_442
79. Piroxicam [inn]
80. Piroxicam [jan]
81. Piroxicam: Form Alpha1
82. Piroxicam: Form Alpha2
83. Piroxicam [mi]
84. Piroxicam [usan]
85. Prestwick0_000211
86. Prestwick1_000211
87. Prestwick2_000211
88. Prestwick3_000211
89. Spectrum2_001287
90. Spectrum3_000780
91. Spectrum4_000968
92. Spectrum5_001445
93. Lopac-p-5654
94. Piroxicam [vandf]
95. Piroxicam Anhydrous
96. Chembl527
97. Piroxicam [mart.]
98. P 5654
99. Piroxicam [usp-rs]
100. Piroxicam [who-dd]
101. Bidd:pxr0154
102. Lopac0_000900
103. Oprea1_714707
104. Schembl13462
105. Schembl21350
106. Bspbio_000221
107. Bspbio_001030
108. Bspbio_002460
109. Kbiogr_000370
110. Kbiogr_001315
111. Kbioss_000370
112. Kbioss_001595
113. Mls000038002
114. Mls001148207
115. Mls001304054
116. Mls004774122
117. Divk1c_000369
118. Spectrum1500491
119. Spbio_001293
120. Spbio_002142
121. Piroxicam (jp17/usp/inn)
122. Bpbio1_000245
123. Gtpl7273
124. Piroxicam, >=98% (tlc)
125. Schembl3703617
126. Piroxicam [orange Book]
127. Piroxicam For System Suitability
128. Chembl1518938
129. Dtxsid5021170
130. Piroxicam [ep Monograph]
131. Bcbcmap01_000176
132. Bdbm85245
133. Hms501c11
134. Kbio1_000369
135. Kbio2_000370
136. Kbio2_001595
137. Kbio2_002938
138. Kbio2_004163
139. Kbio2_005506
140. Kbio2_006731
141. Kbio3_000719
142. Kbio3_000720
143. Kbio3_001680
144. Piroxicam [usp Monograph]
145. Ninds_000369
146. Piroxicam 1.0 Mg/ml In Methanol
147. Bio1_000363
148. Bio1_000852
149. Bio1_001341
150. Bio2_000355
151. Bio2_000835
152. Glxc-26155
153. Glxc-26156
154. Hms1362d11
155. Hms1568l03
156. Hms1792d11
157. Hms1920h22
158. Hms1990d11
159. Hms2089b06
160. Hms2092a05
161. Hms2095l03
162. Hms2231g03
163. Hms3262d22
164. Hms3267i03
165. Hms3369b07
166. Hms3403d11
167. Hms3414h17
168. Hms3429l03
169. Hms3655c04
170. Hms3678h15
171. Hms3712l03
172. Hms3884c08
173. Pharmakon1600-01500491
174. Bcp02919
175. Hy-b0253
176. Nsc_4856
177. Tox21_110231
178. Tox21_200151
179. Tox21_500900
180. Ccg-36403
181. Nsc757284
182. S1713
183. Stk177288
184. Zinc12466469
185. Zinc51133897
186. Zinc87724780
187. Akos000714958
188. Akos025312555
189. Akos026749939
190. Tox21_110231_1
191. Am84917
192. Db00554
193. Ks-5322
194. Lp00900
195. Nsc-757284
196. Sdccgsbi-0050875.p005
197. (4-hydroxy-2-methyl-1,1-dioxobenzo[e]1,2-thiazin-3-yl)-n-(2-pyridyl)carboxamid E
198. (4-hydroxy-2-methyl-1,1-dioxobenzo[e]1,2-thiazin-3-yl)-n-(2-pyridyl)carboxamide
199. 2h-1,2-benzothiazine-3-carboxamide,4-hydroxy-2-methyl-n-2-pyridinyl-, 1,1-dioxide
200. 3,4-dihydro-2-methyl-4-oxo-n-2-pyridyl-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
201. 4-hydroxy-2-methyl-n-(pyridin-2-yl)-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
202. Idi1_000369
203. Idi1_002110
204. Ncgc00015823-01
205. Ncgc00015823-03
206. Ncgc00015823-04
207. Ncgc00015823-05
208. Ncgc00015823-06
209. Ncgc00015823-07
210. Ncgc00015823-08
211. Ncgc00015823-09
212. Ncgc00015823-10
213. Ncgc00015823-11
214. Ncgc00015823-12
215. Ncgc00015823-13
216. Ncgc00015823-14
217. Ncgc00015823-15
218. Ncgc00015823-17
219. Ncgc00015823-18
220. Ncgc00015823-20
221. Ncgc00015823-29
222. Ncgc00021244-03
223. Ncgc00021244-05
224. Ncgc00021244-06
225. Ncgc00021244-07
226. Ncgc00021244-08
227. Ncgc00021244-09
228. Ncgc00188982-01
229. Ncgc00257705-01
230. Ncgc00261585-01
231. Piroxicam 100 Microg/ml In Acetonitrile
232. 1488516-58-0
233. Ac-24190
234. Nci60_022912
235. Bcp0726000299
236. Sbi-0050875.p004
237. Cas_36322-90-4
238. Piroxicam, Meets Usp Testing Specifications
239. Eu-0100900
240. Ft-0630590
241. Ft-0673949
242. P1905
243. Sw219862-1
244. En300-70724
245. A19556
246. C01608
247. D00127
248. D70554
249. Ab00052074-21
250. Ab00052074-22
251. Ab00052074_23
252. Ab00052074_24
253. 322p904
254. Q408676
255. Sr-01000000199-3
256. Sr-01000000199-5
257. Sr-01000000199-9
258. W-106626
259. Sr-01000000199-12
260. F0001-2399
261. Piroxicam, British Pharmacopoeia (bp) Reference Standard
262. Z1259192069
263. Piroxicam, European Pharmacopoeia (ep) Reference Standard
264. Piroxicam, United States Pharmacopeia (usp) Reference Standard
265. Piroxicam, Pharmaceutical Secondary Standard; Certified Reference Material
266. 4-hydroxy-2-methyl-1,1-dioxo-n-(2-pyridyl)-1$l^{6},2-benzothiazine-3-carboxamide
267. 4-hydroxy-2-methyl-1,1-dioxo-n-(pyridin-2-yl)-2h-1$l^{6},2-benzothiazine-3-carboxamide
268. 4-hydroxy-2-methyl-3-(2-pyridylcarbamoyl)-2h-1,2-benzothiazine 1,1-dioxide
269. 4-hydroxy-2-methyl-n-(2-pyridyl)-2h-1,2-benzo-thiazine-3-carboxamide1,1-dioxide
270. 4-hydroxy-2-methyl-n-(pyridin-2-yl)-2h-benzo[e][1,2]thiazine-3-carboxamide1,1-dioxide
271. 4-hydroxy-2-methyl-n-2-pyridinyl-2h-1,2-benzothiazine-3-carboxamide1,1-dioxide
272. N-(2-pyridyl)-4-hydroxy-2-methyl-2h-1,2-benzothiazine-3-carboxamide 1,1-dioxide
273. 1044566-76-8
274. 3-{hydroxy[(pyridin-2-yl)amino]methylidene}-2-methyl-3,4-dihydro-2h-1$l^{6},2-benzothiazine-1,1,4-trione
275. 3-{hydroxy[(pyridin-2-yl)amino]methylidene}-2-methyl-3,4-dihydro-2h-1lambda6,2-benzothiazine-1,1,4-trione
276. 4-hydroxy-2-methyl-1,1-dioxo-n-(2-pyridyl)-1,2-dihydro-1lambda,2-benzothiazine-3-carboxamide
277. 4-hydroxy-2-methyl-n-(2-pyridyl)-2h-1,2-benzothiazine -3-carboxamide-1,1-dioxide Malonic Acid
| Molecular Weight | 331.3 g/mol |
|---|---|
| Molecular Formula | C15H13N3O4S |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 331.06267708 g/mol |
| Monoisotopic Mass | 331.06267708 g/mol |
| Topological Polar Surface Area | 108 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 611 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Akten |
| PubMed Health | Lidocaine (Into the eye) |
| Drug Classes | Anesthetic, Local |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Gel |
| Route | Ophthalmic |
| Strength | 3.5% |
| Market Status | Prescription |
| Company | Akorn |
| 2 of 6 | |
|---|---|
| Drug Name | Feldene |
| PubMed Health | Piroxicam (By mouth) |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | FELDENEcontains piroxicam which is a member of the oxicam group of nonsteroidal anti-inflammatory drugs (NSAIDs). Each maroon and blue capsule contains 10 mg piroxicam, each maroon capsule contains 20 mg piroxicam for oral administration. The chemica... |
| Active Ingredient | Piroxicam |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Pfizer |
| 3 of 6 | |
|---|---|
| Drug Name | Piroxicam |
| PubMed Health | Piroxicam (By mouth) |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Piroxicam capsules USP contain piroxicam which is a member of the oxicam group of non-steroidal anti-inflammatory drugs (NSAIDs). Each dark green and olive capsule contains 10 mg piroxicam, each dark green capsule contains 20 mg piroxicam for oral ad... |
| Active Ingredient | Piroxicam |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Teva; Nostrum Labs; Mutual Pharm; Mylan |
| 4 of 6 | |
|---|---|
| Drug Name | Akten |
| PubMed Health | Lidocaine (Into the eye) |
| Drug Classes | Anesthetic, Local |
| Active Ingredient | Lidocaine hydrochloride |
| Dosage Form | Gel |
| Route | Ophthalmic |
| Strength | 3.5% |
| Market Status | Prescription |
| Company | Akorn |
| 5 of 6 | |
|---|---|
| Drug Name | Feldene |
| PubMed Health | Piroxicam (By mouth) |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | FELDENEcontains piroxicam which is a member of the oxicam group of nonsteroidal anti-inflammatory drugs (NSAIDs). Each maroon and blue capsule contains 10 mg piroxicam, each maroon capsule contains 20 mg piroxicam for oral administration. The chemica... |
| Active Ingredient | Piroxicam |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Pfizer |
| 6 of 6 | |
|---|---|
| Drug Name | Piroxicam |
| PubMed Health | Piroxicam (By mouth) |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Piroxicam capsules USP contain piroxicam which is a member of the oxicam group of non-steroidal anti-inflammatory drugs (NSAIDs). Each dark green and olive capsule contains 10 mg piroxicam, each dark green capsule contains 20 mg piroxicam for oral ad... |
| Active Ingredient | Piroxicam |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 10mg; 20mg |
| Market Status | Prescription |
| Company | Teva; Nostrum Labs; Mutual Pharm; Mylan |
For treatment of osteoarthritis and rheumatoid arthritis.
FDA Label
Piroxicam is in a class of drugs called nonsteroidal anti-inflammatory drugs (NSAIDs). Piroxicam works by reducing hormones that cause inflammation and pain in the body. Piroxicam is used to reduce the pain, inflammation, and stiffness caused by rheumatoid arthritis and osteoarthritis.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
M01AC01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AC - Oxicams
M01AC01 - Piroxicam
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA07 - Piroxicam
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BC - Antiinflammatory agents, non-steroids
S01BC06 - Piroxicam
Absorption
Well absorbed following oral administration.
Route of Elimination
Piroxicam and its biotransformation products are excreted in urine and feces, with about twice as much appearing in the urine as in the feces. Approximately 5% of a piroxicam dose is excreted unchanged. However, a substantial portion of piroxicam elimination occurs by hepatic metabolism. Piroxicam is excreted into human milk.
Volume of Distribution
0.14 L/kg
Renal
Piroxicam has known human metabolites that include 5'-Hydroxypiroxicam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
30 to 86 hours
The antiinflammatory effect of Piroxicam may result from the reversible inhibition of cyclooxygenase, causing the peripheral inhibition of prostaglandin synthesis. The prostaglandins are produced by an enzyme called Cox-1. Piroxicam blocks the Cox-1 enzyme, resulting into the disruption of production of prostaglandins. Piroxicam also inhibits the migration of leukocytes into sites of inflammation and prevents the formation of thromboxane A2, an aggregating agent, by the platelets.








