



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
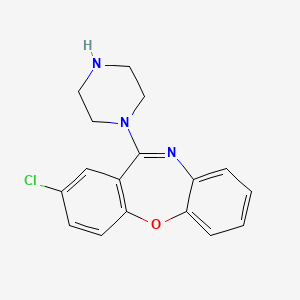

1. 2-chloro-11-(1-piperazinyl)dibenz(b,f)(1,4)oxazepine
2. Asendin
3. Asendis
4. Cl 67,772
5. Cl-67,772
6. Cl67,772
7. Dfanyl
8. Demolox
9. Desmethylloxapine
1. 14028-44-5
2. Asendin
3. Demolox
4. Moxadil
5. Amoxan
6. Desmethylloxapin
7. Amoxapina
8. Amoxapinum
9. Amoxepine
10. Cl-67772
11. Dibenz[b,f][1,4]oxazepine, 2-chloro-11-(1-piperazinyl)-
12. Cl 67772
13. 2-chloro-11-(1-piperazinyl)dibenz(b,f)(1,4)oxazepine
14. 8-chloro-6-piperazin-1-ylbenzo[b][1,4]benzoxazepine
15. Cl-67,772
16. Cl 67,772
17. 2-chloro-11-(piperazin-1-yl)dibenzo[b,f][1,4]oxazepine
18. Asendis
19. Nsc-759559
20. Dibenz(b,f)(1,4)oxazepine, 2-chloro-11-(1-piperazinyl)-
21. Chembl1113
22. 2-chloro-11-(1-piperazinyl)dibenz[b,f][1,4]oxazepine
23. Mls000069371
24. Chebi:2675
25. R63vq857ot
26. 2-chloro-11-piperazin-1-yldibenzo[b,f][1,4]oxazepine
27. Ncgc00015004-09
28. Ascendin
29. Smr000058416
30. Cas-14028-44-5
31. Dsstox_cid_2598
32. Amoxapinum [inn-latin]
33. Dsstox_rid_76652
34. Amoxapina [inn-spanish]
35. Dsstox_gsid_22598
36. 13-chloro-10-(piperazin-1-yl)-2-oxa-9-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,9,12,14-heptaene
37. (e)-2-chloro-11-(piperazin-1-yl)dibenzo[b,f][1,4]oxazepine
38. Asendin (tn)
39. Sr-01000003001
40. Einecs 237-867-1
41. Mfcd00069210
42. Amoxipine
43. Brn 0832057
44. Unii-r63vq857ot
45. 2-chlor-11-(1-piperazinyl)dibenz(b,f)(1,4)oxazepin
46. Amoxapine-[d8]
47. Prestwick_503
48. Amoxapine [usan:usp:inn:ban:jan]
49. Ks-1197
50. 2-chloro-11-(1-piperazinyl)dibenzo[b,f][1,4]oxazepine
51. Opera_id_33
52. Spectrum_000446
53. Cpd000058416
54. Amoxapine [inn]
55. Amoxapine [jan]
56. Amoxapine [mi]
57. Lopac-a-129
58. Amoxapine [usan]
59. Prestwick0_000102
60. Prestwick1_000102
61. Prestwick2_000102
62. Prestwick3_000102
63. Spectrum2_001245
64. Spectrum3_001067
65. Spectrum4_001218
66. Spectrum5_001284
67. Amoxapine [vandf]
68. A-129
69. Amoxapine [mart.]
70. Amoxapine [usp-rs]
71. Amoxapine [who-dd]
72. Lopac0_000116
73. Regid_for_cid_2170
74. Schembl33950
75. Bspbio_000084
76. Bspbio_002654
77. Gtpl201
78. Kbiogr_001656
79. Kbioss_000926
80. Zinc931
81. Maleicacidmonosodiumsalt
82. Divk1c_000236
83. Spectrum2300161
84. Spbio_001150
85. Spbio_002023
86. Amoxapine (jp17/usp/inn)
87. Bpbio1_000094
88. Amoxapine [orange Book]
89. 8-chloro-6-piperazin-1-yl-benzo[b][1,4]benzoxazepine
90. Amoxapine [usp Impurity]
91. Dtxsid7022598
92. Bdbm22870
93. Hms500l18
94. Kbio1_000236
95. Kbio2_000926
96. Kbio2_003494
97. Kbio2_006062
98. Kbio3_001874
99. Amoxapine [usp Monograph]
100. Ninds_000236
101. Hms1568e06
102. Hms2089g10
103. Hms2093n08
104. Hms2095e06
105. Hms2231e13
106. Hms3259g08
107. Hms3260g14
108. Hms3370p03
109. Hms3712e06
110. Pharmakon1600-02300161
111. Hy-b0991
112. Tox21_110064
113. Tox21_302352
114. Tox21_500116
115. Ccg-39135
116. Nsc759559
117. Pdsp1_001609
118. Pdsp2_001593
119. S4218
120. Akos015895092
121. Tox21_110064_1
122. Ac-5495
123. Cs-4485
124. Db00543
125. Lp00116
126. Nc00546
127. Nsc 759559
128. Sdccgsbi-0050104.p004
129. Idi1_000236
130. Ncgc00015004-01
131. Ncgc00015004-02
132. Ncgc00015004-03
133. Ncgc00015004-04
134. Ncgc00015004-05
135. Ncgc00015004-06
136. Ncgc00015004-07
137. Ncgc00015004-08
138. Ncgc00015004-10
139. Ncgc00015004-11
140. Ncgc00015004-13
141. Ncgc00015004-16
142. Ncgc00015004-22
143. Ncgc00023887-03
144. Ncgc00023887-04
145. Ncgc00023887-05
146. Ncgc00023887-06
147. Ncgc00255124-01
148. Ncgc00260801-01
149. Ba164170
150. Sbi-0050104.p003
151. Eu-0100116
152. Ft-0659871
153. Ft-0662122
154. A16406
155. D00228
156. Q58356
157. T70258
158. Ab00052421-13
159. Ab00052421_14
160. Ab00052421_15
161. 028a445
162. A807639
163. L001293
164. J-007373
165. Sr-01000003001-2
166. Sr-01000003001-4
167. Sr-01000003001-6
168. 8-chloro-6-piperazin-1-ylbenzo[b][1,5]benzoxazepine
169. Brd-k02265150-001-05-9
170. Brd-k02265150-001-15-8
171. 2-chloro-11-(1-piperazinyl)dibenz[b,f]-1,4-oxazepine
172. 2-chloro-11-(1-piperazinyl)dibenzo[b,f]-1,4-oxazepine
173. 2-chloro-11-piperazin-1-yl-dibenzo[b,f][1,4]oxazepine
174. 8-chloro-6-(1-piperazinyl)benzo[b][1,4]benzoxazepine
175. Z1551429736
176. 2-chloro-11-(1-piperazinyl)dibenzo[b,f][1,4]oxazepine #
177. 8-chloranyl-6-piperazin-1-yl-benzo[b][1,4]benzoxazepine
178. Amoxapine, 1.0 Mg/ml In Methanol, Certified Reference Material
179. Amoxapine, United States Pharmacopeia (usp) Reference Standard
180. Dibenzo[b,f][1,4]oxazepine, 2-chloro-11-(1-piperazinyl)-
181. Loxapine Succinate Impurity, Amoxapine- [usp Impurity]
182. Amoxapine, Pharmaceutical Secondary Standard; Certified Reference Material
183. 13-chloro-10-(piperazin-1-yl)-2-oxa-9-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3,5,7,9,12,14-heptaene
| Molecular Weight | 313.8 g/mol |
|---|---|
| Molecular Formula | C17H16ClN3O |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 313.0981898 g/mol |
| Monoisotopic Mass | 313.0981898 g/mol |
| Topological Polar Surface Area | 36.9 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 424 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Amoxapine |
| PubMed Health | Amoxapine (By mouth) |
| Drug Classes | Antidepressant |
| Drug Label | Amoxapine is an antidepressant of the dibenzoxazepine class, chemically distinct from the dibenzazepines, dibenzocycloheptenes, and dibenzoxepines.It is designated chemically as 2-Chloro-11-(1-piperazinyl)dibenz[b,f][1,4]oxazepine. The structural for... |
| Active Ingredient | Amoxapine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg; 150mg |
| Market Status | Prescription |
| Company | Watson Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Amoxapine |
| PubMed Health | Amoxapine (By mouth) |
| Drug Classes | Antidepressant |
| Drug Label | Amoxapine is an antidepressant of the dibenzoxazepine class, chemically distinct from the dibenzazepines, dibenzocycloheptenes, and dibenzoxepines.It is designated chemically as 2-Chloro-11-(1-piperazinyl)dibenz[b,f][1,4]oxazepine. The structural for... |
| Active Ingredient | Amoxapine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg; 150mg |
| Market Status | Prescription |
| Company | Watson Labs |
For the relief of symptoms of depression in patients with neurotic or reactive depressive disorders as well as endogenous and psychotic depressions. May also be used to treat depression accompanied by anxiety or agitation.
Amoxapine is a tricyclic antidepressant of the dibenzoxazepine class, chemically distinct from the dibenzodiazepines, dibenzocycloheptenes, and dibenzoxepines. It has a mild sedative component to its action. The mechanism of its clinical action in man is not well understood. In animals, amoxapine reduced the uptake of nor-epinephirine and serotonin and blocked the response of dopamine receptors to dopamine. Amoxapine is not a monoamine oxidase inhibitor. Clinical studies have demonstrated that amoxapine has a more rapid onset of action than either amitriptyline or imipramine
Adrenergic Uptake Inhibitors
Drugs that block the transport of adrenergic transmitters into axon terminals or into storage vesicles within terminals. The tricyclic antidepressants (ANTIDEPRESSIVE AGENTS, TRICYCLIC) and amphetamines are among the therapeutically important drugs that may act via inhibition of adrenergic transport. Many of these drugs also block transport of serotonin. (See all compounds classified as Adrenergic Uptake Inhibitors.)
Antidepressive Agents, Tricyclic
Substances that contain a fused three-ring moiety and are used in the treatment of depression. These drugs block the uptake of norepinephrine and serotonin into axon terminals and may block some subtypes of serotonin, adrenergic, and histamine receptors. However, the mechanism of their antidepressant effects is not clear because the therapeutic effects usually take weeks to develop and may reflect compensatory changes in the central nervous system. (See all compounds classified as Antidepressive Agents, Tricyclic.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
Neurotransmitter Uptake Inhibitors
Drugs that inhibit the transport of neurotransmitters into axon terminals or into storage vesicles within terminals. For many transmitters, uptake determines the time course of transmitter action so inhibiting uptake prolongs the activity of the transmitter. Blocking uptake may also deplete available transmitter stores. Many clinically important drugs are uptake inhibitors although the indirect reactions of the brain rather than the acute block of uptake itself is often responsible for the therapeutic effects. (See all compounds classified as Neurotransmitter Uptake Inhibitors.)
Selective Serotonin Reuptake Inhibitors
Compounds that specifically inhibit the reuptake of serotonin in the brain. (See all compounds classified as Selective Serotonin Reuptake Inhibitors.)
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AA - Non-selective monoamine reuptake inhibitors
N06AA17 - Amoxapine
Absorption
Rapidly and almost completely absorbed from the GI tract. Peak plasma concentrations occur within 1-2 hours of oral administration of a single dose.
Route of Elimination
60-69% of a single orally administered dose of amoxapine is excreted in urine, principally as conjugated metabolites. 7-18% of the dose is excrete feces mainly as unconjugated metabolites. Less than 5% of the dose is excreted as unchanged drug in urine.
Volume of Distribution
Widely distributed in body tissues with highest concentrations found in lungs, spleen, kidneys, heart, and brain. Lower concentrations can be detected in testes and muscle.
Amoxapine is almost completely metabolized in the liver to its major metabolite, 8-hydroxyamoxapine, and a minor metabolite, 7-hydroxyamoxapine. Both metabolites are phamacologically inactive and have half-lives of approximately 30 and 6.5 hours, respectively.
8 hours
Amoxapine acts by decreasing the reuptake of norepinephrine and serotonin (5-HT).




