



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
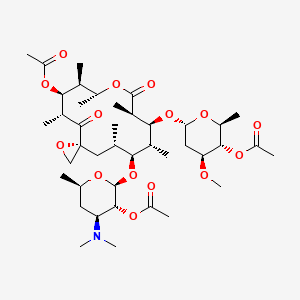

1. Oleandocetin
2. Tao
3. Triacetyloleandomycin
1. Triacetyloleandomycin
2. Oleandomycin Triacetate
3. Oleandocetine
4. Cyclamycin
5. Tribiocillina
6. Oleandomycin Triacetyl Ester
7. 2751-09-9
8. Evramicina
9. Troleandomicina
10. Troleandomycine
11. Triocetin
12. Aovine
13. Troleandomycinum
14. Oleandomycin, Triacetate (ester)
15. Matromycin T
16. Oleandomycin Triacetate Ester
17. Triacetyloleandomycinum
18. Matromicina
19. Treolmicina
20. Oleandomycin (as Troleandomycin)
21. Chebi:45735
22. C4dz64560d
23. Nsc-108166
24. Ai3-50166
25. Tao
26. Triacetyloleandomycin (jan)
27. Triacetyloleandomycin [jan]
28. Oleandomycin, Triacetyl-
29. Triolan
30. Viamicina
31. Wytrion
32. Treis-micina
33. Wy 651
34. Acetyloleandomycin
35. Tao (van)
36. Tao (tn)
37. Prestwick3_000036
38. Troleandomycin [mi]
39. Troleandomycin (usan/inn)
40. Troleandomycin [inn]
41. Bspbio_000131
42. Troleandomycin [usan]
43. Troleandomycine [inn-french]
44. Troleandomycinum [inn-latin]
45. Schembl125071
46. Troleandomycin [vandf]
47. Unii-c4dz64560d
48. Troleandomicina [inn-spanish]
49. Triacetyl Ester Of Oleandomycin
50. Troleandomycin [mart.]
51. Bpbio1_000145
52. Chembl564085
53. T.a.o.
54. Troleandomycin [who-dd]
55. Dtxsid2023721
56. Hms2089b10
57. Hms2095g13
58. Hms3712g13
59. Fmoc-(r)-3-amino-5-hexenoicacid
60. Einecs 220-392-9
61. Troleandomycin [orange Book]
62. Bdbm50370258
63. Lmpk04000042
64. Troleandomycin [usp Impurity]
65. Troleandomycin [usan:usp:inn:ban]
66. Zinc169307271
67. Ccg-220036
68. Db13179
69. Nsc 108166
70. Ncgc00179654-01
71. Hy-108881
72. Ab00513798
73. Cs-0031241
74. D01322
75. Ab00513798-02
76. Oleandomycin (as Troleandomycin) [vandf]
77. Q1087499
78. Brd-k38310698-001-01-9
79. (3r,5r,6s,7r,8r,11r,12s,13r,14s,15s)-12-[(4-o-acetyl-2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)oxy]-14-{[2-o-acetyl-3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl]oxy}-5,7,8,11,13,15-hexamethyl-4,10-dioxo-1,9-dioxaspiro[2.13]hexadecan-6-yl Acetate
80. [(3r,5s,6s,7r,8s,9r,12r,13s,14s,15r)-6-[(2s,3r,4s,6r)-3-acetyloxy-4-(dimethylamino)-6-methyloxan-2-yl]oxy-8-[(2r,4s,5s,6s)-5-acetyloxy-4-methoxy-6-methyloxan-2-yl]oxy-5,7,9,12,13,15-hexamethyl-10,16-dioxo-1,11-dioxaspiro[2.13]hexadecan-14-yl] Acetate
| Molecular Weight | 814.0 g/mol |
|---|---|
| Molecular Formula | C41H67NO15 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 12 |
| Exact Mass | 813.45107043 g/mol |
| Monoisotopic Mass | 813.45107043 g/mol |
| Topological Polar Surface Area | 184 Ų |
| Heavy Atom Count | 57 |
| Formal Charge | 0 |
| Complexity | 1430 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of bacterial infection.
Troleandomycin, like other macrolide antibiotics, inhibits bacterial protein synthesis to prevent growth.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FA - Macrolides
J01FA08 - Troleandomycin
Troleandomycin has known human metabolites that include [(3R,5S,6S,7R,8S,9R,12R,13S,14S,15R)-8-[(2R,4S,5S,6S)-5-acetyloxy-4-methoxy-6-methyloxan-2-yl]oxy-6-[(2S,3R,4S,6R)-3-acetyloxy-6-methyl-4-(methylamino)oxan-2-yl]oxy-5,7,9,12,13,15-hexamethyl-10,16-dioxo-1,11-dioxaspiro[2.13]hexadecan-14-yl] acetate.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
As a macrolide, troleandomycin binds to the 50S subunit of the bacterial ribosome. This binding inhibits translocation of tRNA along the A, P, and E sites of the ribosome. With tRNA unable to move from site to site, amino acids cannot be deposited onto the polypeptide chain leading to failure of protein synthesis. Bacterial cell growth and duplication is inhibited without the ability to generate the necessary proteins.


