



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
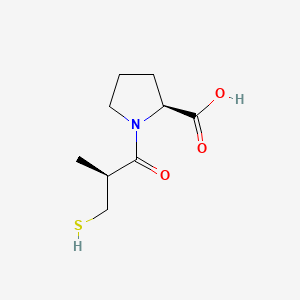

1. (s)-1-(3-mercapto-2-methyl-1-oxopropyl)-l-proline
2. Capoten
3. Lopirin
4. Sq 14,225
5. Sq 14,534
6. Sq 14225
7. Sq 14534
8. Sq-14,225
9. Sq-14,534
10. Sq-14225
11. Sq-14534
12. Sq14,225
13. Sq14,534
14. Sq14225
15. Sq14534
1. 62571-86-2
2. Capoten
3. L-captopril
4. Lopirin
5. Captopryl
6. Garranil
7. Cesplon
8. Captolane
9. Tensoprel
10. Acepress
11. Captoril
12. Dilabar
13. Hypertil
14. Tenosbon
15. Alopresin
16. Captoprilum
17. Acepril
18. Apopril
19. Lopril
20. Acediur
21. Aceplus
22. (2s)-1-[(2s)-2-methyl-3-sulfanylpropanoyl]pyrrolidine-2-carboxylic Acid
23. Sq-14225
24. Farcopril
25. Hypopress
26. Tensiomin
27. Tensobon
28. Zapto
29. Captoprilum [inn-latin]
30. Sq 14225
31. D-2-methyl-3-mercaptopropanoyl-l-proline
32. D-3-mercapto-2-methylpropanoyl-l-proline
33. Sq 14,225
34. 1-((2s)-3-mercapto-2-methylpropionyl)-l-proline
35. Sa 333
36. D-3-mercapto-2-methylpropionylproline
37. Captomax
38. Mepha
39. L-proline, 1-[(2s)-3-mercapto-2-methyl-1-oxopropyl]-
40. 1-[(2s)-2-methyl-3-sulfanylpropanoyl]-l-proline
41. (2s)-1-(3-mercapto-2-methylpropionyl)-l-proline
42. Hipertil
43. Chebi:3380
44. C09aa01
45. L-proline, 1-(3-mercapto-2-methyl-1-oxopropyl)-, (s)-
46. Nsc-757419
47. 9g64rsx1xd
48. Chembl1560
49. 1-[(2s)-3-mercapto-2-methyl-1-oxopropyl]-l-proline
50. Mls000069484
51. Asisten
52. Isopresol
53. (s)-1-((s)-3-mercapto-2-methylpropanoyl)pyrrolidine-2-carboxylic Acid
54. (-)-captopril
55. 1-(3-mercapto-2-methyl-1-oxopropyl)-l-proline
56. Sa-333
57. (s)-1-(3-mercapto-2-methyl-1-oxopropyl)-l-proline
58. L-proline, 1-((2s)-3-mercapto-2-methyl-1-oxopropyl)-
59. Ncgc00023654-04
60. Smr000059061
61. Sq -14225
62. Lopirin [switzerland]
63. 1-pyrrolidinecarboxylic Acid, 1-(d-3-mercapto-2-methyl-1-propionyl)-, L-(s,s)-
64. Dsstox_cid_17197
65. Dsstox_rid_79306
66. Dsstox_gsid_37197
67. Novocaptopril
68. Captril
69. Captopril (capoten)
70. Capozide (salt/mix)
71. 3-mercapto-2-methylpropionyl-proline
72. X8z
73. Apopril (tn)
74. Capoten (tn)
75. Hsdb 6527
76. 1-[(2s)-3-mercapto-2-methylpropionyl]-l-proline
77. Sr-01000075603
78. Einecs 263-607-1
79. Unii-9g64rsx1xd
80. Component Of Captea (salt/mix)
81. Component Of Acezide (salt/mix)
82. Component Of Ecazide (salt/mix)
83. 1-(d-3-mercapto-2-methyl-1-oxopropyl)-l-proline (s,s)
84. Captopril,(s)
85. Prestwick_103
86. Capoten;l-captopril
87. Mfcd00168073
88. Captopril [usan:usp:inn:ban:jan]
89. Sq14534
90. Schembl4
91. Spectrum_000688
92. Captopril [inn]
93. Captopril [jan]
94. Captopril [mi]
95. Captopril [hsdb]
96. Captopril [usan]
97. Opera_id_1041
98. Prestwick3_000019
99. Spectrum2_001211
100. Spectrum3_001388
101. Spectrum4_000811
102. Spectrum5_001587
103. Captopril [vandf]
104. Lopac-c-4042
105. 1-((2s)-2-methyl-3-sulfanylpropanoyl)-l-proline #
106. Captopril [mart.]
107. Epitope Id:114065
108. Upcmld-dp003
109. C 4042
110. Captopril [usp-rs]
111. Captopril [who-dd]
112. Captopril [who-ip]
113. Lopac0_000302
114. Bspbio_000057
115. Bspbio_002976
116. Kbiogr_001321
117. Kbioss_001168
118. Mls001076488
119. Divk1c_000208
120. Spectrum1500682
121. Spbio_001022
122. Captopril (jp17/usp/inn)
123. (s)-1-(3-mercapto-2-methyl-1-oxo-propyl)-l-proline
124. Bpbio1_000063
125. Gtpl5158
126. Captopril [orange Book]
127. Captopril For System Suitability
128. Captopril [ep Monograph]
129. Captopril [usp Impurity]
130. Dtxsid1037197
131. Upcmld-dp003:001
132. Bdbm21642
133. Hms500k10
134. Kbio1_000208
135. Kbio2_001168
136. Kbio2_003736
137. Kbio2_006304
138. Kbio3_002196
139. Zinc57001
140. Captopril [usp Monograph]
141. 1j37
142. Ninds_000208
143. Hms1921c12
144. Hms2089p19
145. Hms2092i12
146. Hms2095c19
147. Hms2233i04
148. Hms3259g10
149. Hms3260n06
150. Hms3712c19
151. Pharmakon1600-01500682
152. Sa333
153. Captoprilum [who-ip Latin]
154. Hy-b0368
155. Tox21_110890
156. Tox21_500302
157. Bbl033600
158. Ccg-39104
159. Ei-213
160. Nsc757419
161. S2051
162. Stk802012
163. Captopril 100 Microg/ml In Methanol
164. Akos005622581
165. Captopril, >=98% (hplc), Powder
166. Tox21_110890_1
167. Bcp9000485
168. Cs-2425
169. Db01197
170. Ks-5025
171. Lp00302
172. Nc00554
173. Nsc 757419
174. Sdccgsbi-0050290.p006
175. Idi1_000208
176. Smp1_000056
177. Ncgc00015235-01
178. Ncgc00015235-02
179. Ncgc00023654-03
180. Ncgc00023654-05
181. Ncgc00023654-06
182. Ncgc00023654-07
183. Ncgc00023654-08
184. Ncgc00023654-09
185. Ncgc00023654-10
186. Ncgc00023654-11
187. Ncgc00023654-13
188. Ncgc00023654-25
189. Ncgc00023654-26
190. Ncgc00260987-01
191. Ac-12047
192. Ac-32120
193. Sbi-0050290.p004
194. Captopril, Meets Usp Testing Specifications
195. Eu-0100302
196. ((s)-3-mercapto-2-methylpropanoyl)-l-proline
197. Bim-0050290.0001
198. C06867
199. D00251
200. Ab00052156-16
201. Ab00052156_17
202. Ab00052156_18
203. 571c862
204. Q421119
205. Sr-01000000039
206. 1-[(2s)-3-mercapto-2-methylpropionyl]- L-proline
207. Sr-01000000039-2
208. Sr-01000075603-1
209. Sr-01000075603-3
210. 1-[(s)-3-mercapto-2-methyl-1-oxopropyl]-l-proline
211. Brd-k54529596-001-04-0
212. Brd-k54529596-001-15-6
213. Captopril, British Pharmacopoeia (bp) Reference Standard
214. Z2786051697
215. Captopril, European Pharmacopoeia (ep) Reference Standard
216. Captopril, United States Pharmacopeia (usp) Reference Standard
217. (s)-1-((s)-3-mercapto-2-methylpropanoyl)pyrrolidine-2-carboxylicacid
218. Captopril, Pharmaceutical Secondary Standard; Certified Reference Material
219. L-?proline, 1-?[(2s)?-?3-?mercapto-?2-?methyl-?1-?oxopropyl]?-
220. Captopril For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 217.29 g/mol |
|---|---|
| Molecular Formula | C9H15NO3S |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 217.07726451 g/mol |
| Monoisotopic Mass | 217.07726451 g/mol |
| Topological Polar Surface Area | 58.6 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 244 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Capoten |
| PubMed Health | Captopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Captopril is a specific competitive inhibitor of angiotensin I-converting enzyme (ACE), the enzyme responsible for the conversion of angiotensin I to angiotensin II.Captopril is designated chemically as 1-[(2S)-3-mercapto-2-methylpropionyl]-L-proline... |
| Active Ingredient | Captopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg; 12.5mg |
| Market Status | Prescription |
| Company | Par Pharm |
| 2 of 4 | |
|---|---|
| Drug Name | Captopril |
| PubMed Health | Captopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Active Ingredient | Captopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg; 12.5mg |
| Market Status | Prescription |
| Company | Watson Labs; Wockhardt; Teva; Apotex; Sandoz; Hikma Pharms; Mylan; Stason |
| 3 of 4 | |
|---|---|
| Drug Name | Capoten |
| PubMed Health | Captopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Captopril is a specific competitive inhibitor of angiotensin I-converting enzyme (ACE), the enzyme responsible for the conversion of angiotensin I to angiotensin II.Captopril is designated chemically as 1-[(2S)-3-mercapto-2-methylpropionyl]-L-proline... |
| Active Ingredient | Captopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg; 12.5mg |
| Market Status | Prescription |
| Company | Par Pharm |
| 4 of 4 | |
|---|---|
| Drug Name | Captopril |
| PubMed Health | Captopril (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Active Ingredient | Captopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 25mg; 50mg; 12.5mg |
| Market Status | Prescription |
| Company | Watson Labs; Wockhardt; Teva; Apotex; Sandoz; Hikma Pharms; Mylan; Stason |
Angiotensin-Converting Enzyme Inhibitors; Antihypertensive Agents
National Library of Medicine's Medical Subject Headings. Captopril. Online file (MeSH, 2017). Available from, as of August 30, 2017: https://www.nlm.nih.gov/mesh/2017/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Captopril is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of August 30, 2017: https://clinicaltrials.gov/
Captopril tablets are indicated for the treatment of hypertension. ... Captopril tablets are effective alone and in combination with other antihypertensive agents, especially thiazide-type diuretics. The blood pressure lowering effects of captopril and thiazides are approximately additive. /Included in US product label/
NIH; DailyMed. Current Medication Information for Captopril (Captopril Tablet) (Updated: August 2017). Available from, as of October 27, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5599d8f8-efc8-4acc-a507-280b89fd1dda
Captopril tablets are indicated in the treatment of congestive heart failure usually in combination with diuretics and digitalis. The beneficial effect of captopril in heart failure does not require the presence of digitalis, however, most controlled clinical trial experience with captopril has been in patients receiving digitalis, as well as diuretic treatment. /Included in US product label/
NIH; DailyMed. Current Medication Information for Captopril (Captopril Tablet) (Updated: August 2017). Available from, as of October 27, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5599d8f8-efc8-4acc-a507-280b89fd1dda
For more Therapeutic Uses (Complete) data for Captopril (11 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue captopril tablets as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
NIH; DailyMed. Current Medication Information for Captopril (Captopril Tablet) (Updated: August 2017). Available from, as of October 27, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5599d8f8-efc8-4acc-a507-280b89fd1dda
Captopril is generally well tolerated in most patients; however, serious adverse effects (e.g., neutropenia, agranulocytosis, proteinuria, aplastic anemia) have been reported rarely, mainly in patients with renal impairment (especially those with collagen vascular disease). Captopril- induced adverse effects are often alleviated by dosage reduction, occasionally disappear despite continued treatment and without dosage reduction, and are usually reversible following discontinuance of the drug. The most common adverse effects of captopril are rash and loss of taste perception. Adverse effects requiring discontinuance of captopril therapy occur in about 4-12% of patients.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2074
Captopril is contraindicated in patients with known hypersensitivity to the drug or to another angiotension-converting enzyme inhibitor (eg, those who experienced angioedema during therapy with another angiotension-converting enzyme inhibitor).
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2077
Patients receiving captopril should be warned not to interrupt or discontinue therapy unless instructed by their physician. Patients with congestive heart failure receiving captopril should be cautioned against rapid increases in physical activity.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2077
For more Drug Warnings (Complete) data for Captopril (32 total), please visit the HSDB record page.
A case of a 75 year old male who committed suicide by taking an overdose of captopril was reported. He took approximately ninety 12.5 mg captopril tablets. ...
PMID:2231837 Park H et al; J Toxicol Clin Toxicol 28 (3): 379-82 (1990)
For the treatment of essential or renovascular hypertension (usually administered with other drugs, particularly thiazide diuretics). May be used to treat congestive heart failure in combination with other drugs (e.g. cardiac glycosides, diuretics, β-adrenergic blockers). May improve survival in patients with left ventricular dysfunction following myocardial infarction. May be used to treat nephropathy, including diabetic nephropathy.
Treatment of heart failure
Captopril, an ACE inhibitor, antagonizes the effect of the RAAS. The RAAS is a homeostatic mechanism for regulating hemodynamics, water and electrolyte balance. During sympathetic stimulation or when renal blood pressure or blood flow is reduced, renin is released from the granular cells of the juxtaglomerular apparatus in the kidneys. In the blood stream, renin cleaves circulating angiotensinogen to ATI, which is subsequently cleaved to ATII by ACE. ATII increases blood pressure using a number of mechanisms. First, it stimulates the secretion of aldosterone from the adrenal cortex. Aldosterone travels to the distal convoluted tubule (DCT) and collecting tubule of nephrons where it increases sodium and water reabsorption by increasing the number of sodium channels and sodium-potassium ATPases on cell membranes. Second, ATII stimulates the secretion of vasopressin (also known as antidiuretic hormone or ADH) from the posterior pituitary gland. ADH stimulates further water reabsorption from the kidneys via insertion of aquaporin-2 channels on the apical surface of cells of the DCT and collecting tubules. Third, ATII increases blood pressure through direct arterial vasoconstriction. Stimulation of the Type 1 ATII receptor on vascular smooth muscle cells leads to a cascade of events resulting in myocyte contraction and vasoconstriction. In addition to these major effects, ATII induces the thirst response via stimulation of hypothalamic neurons. ACE inhibitors inhibit the rapid conversion of ATI to ATII and antagonize RAAS-induced increases in blood pressure. ACE (also known as kininase II) is also involved in the enzymatic deactivation of bradykinin, a vasodilator. Inhibiting the deactivation of bradykinin increases bradykinin levels and may sustain its effects by causing increased vasodilation and decreased blood pressure.
Angiotensin-Converting Enzyme Inhibitors
A class of drugs whose main indications are the treatment of hypertension and heart failure. They exert their hemodynamic effect mainly by inhibiting the renin-angiotensin system. They also modulate sympathetic nervous system activity and increase prostaglandin synthesis. They cause mainly vasodilation and mild natriuresis without affecting heart rate and contractility. (See all compounds classified as Angiotensin-Converting Enzyme Inhibitors.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
C09AA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09A - Ace inhibitors, plain
C09AA - Ace inhibitors, plain
C09AA01 - Captopril
Absorption
60-75% in fasting individuals; food decreases absorption by 25-40% (some evidence indicates that this is not clinically significant)
The drug /captopril/ is metabolized and renally excreted. More than 95% of a dose is excreted renally, both as unchanged (45-50%) drug and as metabolites.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 206
In dogs, approximately 75% of an oral dose is absorbed but food in the GI tract reduces bioavailability by 30-40%. It is distributed to most tissues (not the CNS) and is 40% bound to plasma proteins in dogs.
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 206
Approximately 60-75% of an oral dose of captopril is rapidly absorbed from the GI tract in fasting healthy individuals or hypertensive patients. Food may decrease absorption of captopril by up to 25-40%, although there is some evidence that this effect is not clinically important. Following oral administration of a single 100-mg dose of captopril in fasting healthy individuals in one study, average peak blood drug concentrations of 800 ng/mL were attained in 1 hour.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2079
/MILK/ Concentrations of captopril in human milk are approximately one percent of those in maternal blood.
NIH; DailyMed. Current Medication Information for Captopril (Captopril Tablet) (Updated: August 2017). Available from, as of October 27, 2017: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5599d8f8-efc8-4acc-a507-280b89fd1dda
For more Absorption, Distribution and Excretion (Complete) data for Captopril (7 total), please visit the HSDB record page.
Hepatic. Major metabolites are captopril-cysteine disulfide and the disulfide dimer of captopril. Metabolites may undergo reversible interconversion.
About half the absorbed dose of captopril is rapidly metabolized, mainly to captopril-cysteine disulfide and the disulfide dimer of captopril. In vitro studies suggest that captopril and its metabolites may undergo reversible interconversions. It has been suggested that the drug may be more extensively metabolized in patients with renal impairment than in patients with normal renal function.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2079
2 hours
A 43 year old patient with mild heart failure attempted suicide by ingesting between 5000 and 7500 mg of captopril. Blood pressure oscillated around 100-120/50-75 mm Hg and pulse rate showed no tendency to accelerate (75-100/min). ... The calculated half-life of captopril was 4.4 hr. ...
PMID:2148447 Lechleitner P et al; Toxicology 64 (3): 325-9 (1990)
The half life of captopril is about 2.8 hours in dogs ... .
Plumb D.C. Veterinary Drug Handbook. 8th ed. (pocket). Ames, IA: Wiley-Blackwell, 2015., p. 206
The elimination half-life of unchanged captopril appears to be less than 2 hours in patients with normal renal function. The elimination half-life of captopril and its metabolites is correlated with creatinine clearance and increases to about 20-40 hours in patients with creatinine clearances less than 20 mL/minute and as long as 6.5 days in anuric patients.
American Society of Health-System Pharmacists 2017; Drug Information 2017. Bethesda, MD. 2017, p. 2079
There are two isoforms of ACE: the somatic isoform, which exists as a glycoprotein comprised of a single polypeptide chain of 1277; and the testicular isoform, which has a lower molecular mass and is thought to play a role in sperm maturation and binding of sperm to the oviduct epithelium. Somatic ACE has two functionally active domains, N and C, which arise from tandem gene duplication. Although the two domains have high sequence similarity, they play distinct physiological roles. The C-domain is predominantly involved in blood pressure regulation while the N-domain plays a role in hematopoietic stem cell differentiation and proliferation. ACE inhibitors bind to and inhibit the activity of both domains, but have much greater affinity for and inhibitory activity against the C-domain. Captopril, one of the few ACE inhibitors that is not a prodrug, competes with ATI for binding to ACE and inhibits and enzymatic proteolysis of ATI to ATII. Decreasing ATII levels in the body decreases blood pressure by inhibiting the pressor effects of ATII as described in the Pharmacology section above. Captopril also causes an increase in plasma renin activity likely due to a loss of feedback inhibition mediated by ATII on the release of renin and/or stimulation of reflex mechanisms via baroreceptors. Captoprils affinity for ACE is approximately 30,000 times greater than that of ATI.
The local role of the renin angiotensin system (RAS) was documented recently beside its conventional systemic functions. Studies showed that the effector angiotensin II (AngII) alters bone health, while inhibition of the angiotensin converting enzyme (ACE-1) preserved these effects. The newly identified Ang1-7 exerts numerous beneficial effects opposing the AngII. Thus, the current study examines the role of Ang1-7 in mediating the osteo-preservative effects of ACEI (captopril) through the G-protein coupled Mas receptor using an ovariectomized (OVX) rat model of osteoporosis. 8 weeks after the surgical procedures, captopril was administered orally (40 mg/kg/day), while the specific Mas receptor blocker (A-779) was delivered at infusion rate of 400 ng/kg/1 min for 6 weeks. Bone metabolic markers were measured in serum and urine. Minerals concentrations were quantified in serum, urine and femoral bones by inductive coupled plasma mass spectroscopy (ICP-MS). Trabecular and cortical morphometry was analyzed in the right distal femurs using micro-CT. Finally, the expressions of RAS peptides, enzymes and receptors along with the receptor activator of NF-kappaB ligand (RANKL) and osteoprotegerin (OPG) were determined femurs heads. OVX animals markedly showed altered bone metabolism and mineralization along with disturbed bone micro-structure. Captopril significantly restored the metabolic bone bio-markers and corrected Ca2+ and P values in urine and bones of estrogen deficient rats. Moreover, the trabecular and cortical morphometric features were repaired by captopril in OVX groups. Captopril also improved the expressions of ACE-2, Ang1-7, Mas and OPG, while abolished OVX-induced up-regulation of ACE-1, AngII, Ang type 1 receptor (AT1R) and RANKL. Inhibition of Ang1-7 cascade by A-779 significantly eradicated captopril protective effects on bone metabolism, mineralization and micro-structure. A-779 also restored OVX effects on RANKL expression and ACE-1/AngII/AT1R cascade and down-regulated OPG expression and ACE-2/Ang1-7/Mas pathway. In line with the clinical observations of the bone-preservative properties following ACE-1 inhibition, local activation of ACE-2/Ang1-7/Mas signaling and suppressed osteoclastogenesis seem responsible for the osteo-preservative effect of captopril, which could offers a potential therapeutic value in treatment of disabling bone and skeletal muscular diseases.
PMID:28531801 Abuohashish HM et al; Biomed Pharmacother 92: 58-68 (2017)






