


API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Foy
2. Gabexate Mesilate
3. Gabexate Mesylate
4. Gabexate Methanesulfonate
5. Gabexate Monomethanesulfonate
6. Gabexate Monomethanesulfonate, 14c Labeled Cpd
7. Gabexate Monomethanesulfonate, 14c-labeled Cpd
8. Mesilate, Gabexate
9. Mesylate, Gabexate
10. Methanesulfonate, Gabexate
11. Monomethanesulfonate, Gabexate
1. 39492-01-8
2. Gabexate [inn]
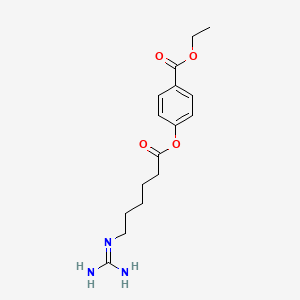
3. Ethyl 4-[6-(diaminomethylideneamino)hexanoyloxy]benzoate
4. Chembl87563
5. Ethyl P-hydroxybenzoate 6-guanidinohexanoate
6. Gabexate (inn)
7. 4v7m9137x9
8. Gabexato
9. Gabexatum
10. Gabexatum [inn-latin]
11. Gabexato [inn-spanish]
12. Methanesulfonic Acid Ethyl 4-[(6-carbamimidamidohexanoyl)oxy]benzoate
13. Unii-4v7m9137x9
14. Gabexate [jan]
15. Gabexate [mi]
16. Prestwick0_001008
17. Prestwick1_001008
18. Prestwick2_001008
19. Prestwick3_001008
20. Gabexate [who-dd]
21. Benzoic Acid, 4-((6-((aminoiminomethyl)amino)-1-oxohexyl)oxy)-, Ethyl Ester
22. Bspbio_001135
23. Schembl446024
24. Spbio_003016
25. Bpbio1_001249
26. Gtpl7863
27. Dtxsid9048566
28. Schembl13287301
29. Chebi:93036
30. Zinc2002226
31. Bdbm50104435
32. Db12831
33. Ethyl 4-(6-guanidinohexanoyloxy)benzoate
34. Ncgc00025297-01
35. Ncgc00025297-02
36. Ncgc00025297-10
37. Ls-14525
38. Sbi-0207080.p001
39. Ethyl 4-((6-guanidinohexanoyl)oxy)benzoate
40. Ethyl-p-(6-guanidinohex-anoyloxy)-benzoate
41. Ab00513998
42. D08004
43. Ab00513998_02
44. Q5515384
45. 4-(6-guanidino-hexanoyloxy)-benzoic Acid Ethyl Ester
46. Brd-k59256312-066-03-3
47. Ethyl 4-({6-[(diaminomethylidene)amino]hexanoyl}oxy)benzoate
48. 4-[6-(diaminomethylideneamino)-1-oxohexoxy]benzoic Acid Ethyl Ester
| Molecular Weight | 321.37 g/mol |
|---|---|
| Molecular Formula | C16H23N3O4 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 11 |
| Exact Mass | 321.16885622 g/mol |
| Monoisotopic Mass | 321.16885622 g/mol |
| Topological Polar Surface Area | 117 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 400 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
No approved indication.
Gabexate bind and inhibits serine proteases in the coagulation cascade to prevent blood clotting. It also prevents proteolytic destruction of IkappaB resulting in suppression of the nuclear factor kappa-B signalling pathway. Ultimately this decreases the production of inflammatory cytokines such as tumor necrosis factor alpha which are produced as a result of NFkappaB activation.
Anticoagulants
Agents that prevent BLOOD CLOTTING. (See all compounds classified as Anticoagulants.)
Serine Proteinase Inhibitors
Exogenous or endogenous compounds which inhibit SERINE ENDOPEPTIDASES. (See all compounds classified as Serine Proteinase Inhibitors.)
Gabexate inhibits kallikrein, plasmin, and thrombin by binding to their active sites. The inhibition of these components of the coagulation cascade ultimately prevents the formation of fibrin which must be present and polymerized to form a clot. Gabexate decreases the production of inflammatory cytokines by attenuating NFkappaB and c-Jun N-terminal kinase (JNK) pathway activity. The exact mechanism for this is unknown but it is thought that gabexate prevents the proteolyytic destruction of IkappaB which deactivates NFkappaB and interferes with activator protein 1 binding to DNA.


