



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
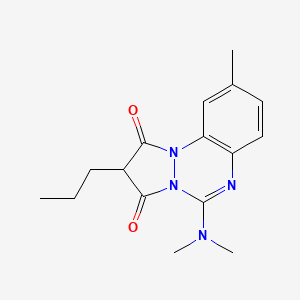

1. 5-(dimethylamino)-9-methyl-2-propyl-1h-pyrazolo(1,2-a)(1,2,4)benzotriazine-1,3(2h)-dione
2. Apazone
3. Apazone Dihydrate
4. Dihydrate, Apazone
5. Prolixan
6. Rheumox
7. Tolyprin
1. Apazone
2. Cinnopropazone
3. Prolixan
4. Azapropazonum
5. Azapropazona
6. 13539-59-8
7. Cinnamin
8. Rheumox
9. Azapropazon
10. Mitrolan
11. Sinnamin
12. Prolix
13. Xani
14. Azapropazone (anhydrous)
15. Ahr-3018
16. Apazone [usan]
17. Prolixan 300
18. Nsc-102824
19. Mi 85
20. Ahr 3018
21. Mi-85
22. Azapropazone Dihydrate
23. Azapropazon [german]
24. Nsc 102824
25. Azapropazone [inn]
26. 5-(dimethylamino)-9-methyl-2-propyl-1h-pyrazolo(1,2-a)(1,2,4)benzotriazine-1,3(2h)-dione
27. 3-dimethylamino-7-methyl-1,2-(n-propylmalonyl)-1,2-dihydro-1,2,4-benzotriazine
28. K2vot966zi
29. 1,2-dihydro-3-dimethylamino-7-methyl-1,2-(propylmalonyl)-1,2,4-benzotriazine
30. 1h-pyrazolo(1,2-a)(1,2,4)benzotriazine-1,3(2h)-dione, 5-(dimethylamino)-9-methyl-2-propyl-
31. 5-(dimethylamino)-9-methyl-2-propyl-1h-pyrazolo[1,2-a][1,2,4]benzotriazine-1,3(2h)-dione
32. Mls002703748
33. Chebi:38010
34. Apazone (usan)
35. Azapropazone (inn)
36. Nsc102824
37. Azapropazon (german)
38. 1h-pyrazolo[1,2-a][1,2,4]benzotriazine-1,3(2h)-dione, 5-(dimethylamino)-9-methyl-2-propyl-
39. 5-(dimethylamino)-9-methyl-2-propylpyrazolo[1,2-a][1,2,4]benzotriazine-1,3-dione
40. Ahr-3018;nsc-102824;nsc102824;
41. Mi-85di
42. Azapropazonum [inn-latin]
43. Azapropazona [inn-spanish]
44. 5-(dimethylamino)-9-methyl-2-propyl-1h-benzo[e]pyrazolo[1,2-a][1,2,4]triazine-1,3(2h)-dione
45. Smr001233401
46. Einecs 236-913-8
47. Unii-k2vot966zi
48. Brn 0623763
49. Ncgc00016701-01
50. Prolixan (tn)
51. Cas-13539-59-8
52. Tolyprin (salt/mix)
53. Apazone [mi]
54. Prestwick0_001003
55. Prestwick1_001003
56. Prestwick2_001003
57. Prestwick3_001003
58. Schembl3190
59. Dsstox_cid_23735
60. Dsstox_rid_80859
61. Nciopen2_007171
62. Dsstox_gsid_45408
63. Azapropazone [mart.]
64. Bspbio_001125
65. Mls002154093
66. (+/-)-apazone
67. Apazone Dihydrate (salt/mix)
68. Azapropazone [who-dd]
69. Spbio_003006
70. Bpbio1_001239
71. Apazone, (+/-)-
72. Chembl1565476
73. Dtxsid6045408
74. Hms1571i07
75. Hms2098i07
76. Hms2231l14
77. Hms3372m10
78. Hms3715i07
79. Tox21_110569
80. Akos015914210
81. Ccg-221003
82. Ncgc00179297-01
83. Ncgc00179297-04
84. 1,2-(propylmalonyl)-1,2,4-benzotriazine
85. Hy-116442
86. Ab00513995
87. Cs-0065495
88. D02966
89. Q368222
90. Sr-01000838825
91. Sr-01000838825-2
92. Wln: T B566 Bnv Evn Hn Dhj D3 Gn1&1 L1
93. 3-dimethylamino-7-methyl-1,2-dihydro-1,2,4-benzotriazine
94. 3-dimethylamino-7-methyl-1-2-(n-propylmalonyl)-1,2,4-benzotriazine
95. 1,2-dihydro-3-(dimethylamino)-7-methyl-1,2-(propylmalonyl)-1,2,4-benzotriazine
96. 1h-pyrazolo[1,2,4]benzotriazine-1,3(2h)-dione, 5-(dimethylamine)-9-methyl-2-propyl-
97. 1h-pyrazolo[1,2,4]benzotriazine-1,3(2h)-dione, 5-(dimethylamino)-9-methyl-2-propyl-
98. 5-(dimethylamino)-9-methyl-2-propyl-1h-pyrazolo[1,2,4]benzotriazine-1,3(2h)-dione
99. 5-(dimethylamino)-9-methyl-2-propyl-1h-pyrazolo[1,2,4]benzotriazine-1,3(2h)dione
100. 5-(dimethylamino)-9-methyl-2-propyl-pyrazolo[1,2-a][1,2,4]benzotriazine-1,3-dione
101. 1h-pyrazolo(1,2-a)(1,2,4)benzotriazine-1,3(2h)-dione, 5-(dimethylamine)-9-methyl-2-propyl-
102. 5-dimethylamino-9-methyl-2-propylpyrazolo(1,2-a)(1,2,4)benzotriazine-1,3(2h)-dione
| Molecular Weight | 300.36 g/mol |
|---|---|
| Molecular Formula | C16H20N4O2 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 300.15862589 g/mol |
| Monoisotopic Mass | 300.15862589 g/mol |
| Topological Polar Surface Area | 56.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 516 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Uricosuric Agents
Gout suppressants that act directly on the renal tubule to increase the excretion of uric acid, thus reducing its concentrations in plasma. (See all compounds classified as Uricosuric Agents.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AX - Other antiinflammatory and antirheumatic agents, non-steroids
M01AX04 - Azapropazone


