



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Lysocline
2. Metacycline
3. Methacycline Monohydrochloride
4. Methyleneoxytetracycline
5. Monohydrochloride, Methacycline
6. Physiomycine
7. Rondomycin
1. Metacycline
2. 914-00-1
3. Rondomycin
4. Methylenecycline
5. Tri-methacycline
6. Methacyclinum
7. 6-methyleneoxytetracycline
8. Metacyclinum
9. Metaciclina
10. 6-methylene-5-oxytetracycline
11. 6-methylene-5-hydroxytetracycline
12. Gs-2876
13. 6-deoxy-6-demethyl-6-methylene-5-oxytetracycline
14. Bialatan
15. 6-demethyl-6-deoxy-5-hydroxy-6-methylenetetracycline
16. Methacycline Base
17. Metacycline (inn)
18. Metacycline [inn]
19. Methacycline Amphoteric
20. Motc
21. Chebi:6805
22. Methacycline (usan)
23. Ir235i7c5p
24. 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-2-naphthacenecarboxamide
25. Oxytetracycline, 6-methylene-
26. Methacycline [usan]
27. (4s,4ar,5s,5ar,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methylidene-3,12-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide
28. Metacyclinum [inn-latin]
29. Methacycline [usan:ban]
30. Metaciclina [inn-spanish]
31. (4s,4ar,5s,5ar,12as)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
32. 2-naphthacenecarboxamide,4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-, (4s,4ar,5s,5ar,12as)-
33. Gs 2876
34. Hsdb 3118
35. Einecs 213-017-5
36. Unii-ir235i7c5p
37. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-, (4s-(4alpha,4aalpha,5alpha,5aalpha,12aalpha))-
38. Spectrum_000381
39. Prestwick0_000964
40. Prestwick1_000964
41. Prestwick2_000964
42. Prestwick3_000964
43. Spectrum2_001398
44. Spectrum3_000917
45. Spectrum4_001016
46. Spectrum5_001576
47. Methacycline [mi]
48. Methacycline [hsdb]
49. Schembl4014
50. Schembl4015
51. Methacycline [vandf]
52. Bspbio_000967
53. Doxycycline Impurity B
54. Kbiogr_001511
55. Kbioss_000861
56. Metacycline [who-dd]
57. Methacycline [mart.]
58. (4s,4ar,5s,5ar,12as)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methylidene-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
59. Divk1c_000591
60. Spbio_001416
61. Spbio_002888
62. Bpbio1_001065
63. Chembl249837
64. Dtxsid2023272
65. Schembl19555132
66. Kbio1_000591
67. Kbio2_000861
68. Kbio2_003429
69. Kbio2_005997
70. Kbio3_001894
71. Ninds_000591
72. Kuc106428n
73. 2-naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-, (4s-(4.alpha.,4a.alpha.,5.alpha.,5a.alpha.,12a.alpha.))-
74. Bcp24961
75. Ksc-12-231a
76. Zinc4019716
77. Bdbm50103629
78. Bdbm50565697
79. Zinc85650610
80. Akos015961140
81. Zinc100017626
82. Db00931
83. Idi1_000591
84. Ncgc00179358-01
85. Ncgc00179358-03
86. Ncgc00179358-05
87. Ac-13213
88. Sbi-0051637.p002
89. Ab00514707
90. C07654
91. D04972
92. Ab00053593-03
93. Ab00053593_04
94. Ab00053593_05
95. Doxycycline Hyclate Impurity B [ep Impurity]
96. Q2365033
97. Doxycycline Monohydrate Impurity B [ep Impurity]
98. (4s,4ar,5s,5ar,12as)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methylene-1,11-dioxo-4,4a,5,5a-tetrahydrotetracene-2-carboxamide
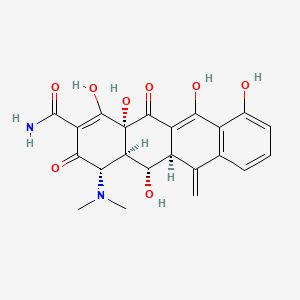
| Molecular Weight | 442.4 g/mol |
|---|---|
| Molecular Formula | C22H22N2O8 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 2 |
| Exact Mass | 442.13761566 g/mol |
| Monoisotopic Mass | 442.13761566 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 998 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antibiotics, Tetracycline
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
The tetracyclines and chloramphenicol are effective and may be lifesaving in rickettsial infections, including Rocky Mountain spotted fever, recrudescent epidemic typhus (Brill's disease), murine typhus, scrub typhus, rickettsialpox, and Q fever. /Tetracyclines/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1123
...ONLY ADVANTAGE OF METHACYCLINE IS ITS LONGER DURATION OF ACTION. ... LOW RATE OF EXCRETION RESULTS IN LOWER URINE CONCN...SO THAT IT IS LESS EFFECTIVE IN URINARY TRACT INFECTIONS THAN SHORTER-ACTING TETRACYCLINES.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1141
METHACYCLINE WAS HIGHLY ACTIVE AGAINST STAPHYLOCOCCUS, E COLI, PROTEUS, & PSEUDOMONAS AERUGINOSA IN VITRO. ITS CHEMOTHERAPEUTIC EFFECT IN MICE WITH EXPTL PNEUMONIA WAS GREATER THAN OF OXYTETRACYCLINE. MEAN CURATIVE DOSE WAS 1.5 TIMES LOWER THAN OXYTETRACYCLINE.
BODUNKOVA LE ET AL; ANTIBIOTIKI (MOSCOW) 20 (11): 1014-18 (1975)
For more Therapeutic Uses (Complete) data for METHACYCLINE (10 total), please visit the HSDB record page.
METHACYCLINE SHOULD NOT BE USED IN PT WITH IMPAIRED RENAL FUNCTION, BUT IF ITS USE CANNOT BE AVOIDED, DOSE-INTERVAL MUST BE INCR TO AS LONG AS 3-4 DAYS IN ANURIA. NONSYSTEMIC ANTACIDS & FOOD INTERFERE WITH ABSORPTION. /METHACYCLINE HYDROCHLORIDE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1141
Pregnant women appear to be particularly susceptible to sever, tetracycline induced hepatic damage. Jaundice appears first, and azotemia, acidosis, and irreversible shock may follow. The live is diffusely infiltrated with fat; although hepatic fat is increased during pregnancy, the quantity appears to be even greater after exposure to a tetracycline. /Tetracyclines/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1121
IT SHOULD BE EMPHASIZED THAT CROSS-SENSITIZATION AMONG VARIOUS TETRACYCLINES IS VERY COMMON IF NOT UNIVERSAL. /TETRACYCLINES/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1122
Demeclocycline, doxycycline, and, to a lesser extent, other derivatives may produce mild-to-severe reactions in the skin of treated individuals exposed to sunlight. /Tetracyclines/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1121
For more Drug Warnings (Complete) data for METHACYCLINE (9 total), please visit the HSDB record page.
2-3. 2= SLIGHTLY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 5-15 G/KG, BETWEEN 1 PINT & 1 QT FOR 70 KG PERSON (150 LB). 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT (OR 1 LB) FOR 70 KG PERSON (150 LB). /TETRACYCLINES/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-172
For the treatment of acute bacterial exacerbations of chronic bronchitis
Methacycline is a tetracycline antibiotic. Similar to other tetracyclines, it has a wide spectrum of antimicrobial action. It is active against most Gram-positive bacteria (pneumococci, streptococci, staphylococci) and Gram-negative bacteria (E. coli, salmonella, shigella, etc.), and towards agents causing onithosis, psittacosis, trachoma, and some Protozoa. Like other tetracyclines, the general usefulness of methacycline has been reduced with the onset of bacterial resistance.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01A - Tetracyclines
J01AA - Tetracyclines
J01AA05 - Metacycline
Absorption
58% absorbed
MOST OF THE TETRACYCLINES ARE ADEQUATELY BUT INCOMPLETELY ABSORBED FROM GI TRACT. ... MOST ABSORPTION TAKES PLACE FROM STOMACH & UPPER SMALL INTESTINE & IS GREATER IN FASTING STATE. IT IS MUCH LESS COMPLETE FROM LOWER PORTION OF INTESTINAL TRACT. /TETRACYCLINES/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1119
ABOUT 50% OF METHACYCLINE IS EXCRETED IN UNCHANGED FORM IN URINE.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1120
VOL OF DISTRIBUTION OF TETRACYCLINES IS RELATIVELY LARGER THAN THAT OF BODY WATER, INDICATING SEQUESTRATION IN SOME TISSUES. THEY ARE BOUND TO PLASMA PROTEINS IN VARYING DEGREE. APPROX VALUES...METHACYCLINE, ABOUT 80%...
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1119
BINDING OF SEVERAL TETRACYCLINES TO HUMAN SERUM ALBUMIN WAS STUDIED USING DIFFERENCE SPECTROPHOTOMETRY & A SPECTROPHOTOMETRIC PROBE, 2-(4-HYDROXYBENZENEAZO)BENZOIC ACID. DRUG-PROBE DISPLACEMENT STUDIES SHOWED THAT METHACYCLINE GAVE GREATEST PROBE DISPLACEMENT.
PMID:3641 ZIA H, PRICE JC; J PHARM SCI 65 (FEB): 226-30 (1976)
CONCN OF METHACYCLINE IN KIDNEYS, LIVER & LUNGS CORRESPONDED TO ITS LEVELS IN BLOOD OF DOGS FOLLOWING ENTERAL ADMIN & WERE 3 TIMES HIGHER THAN OXYTETRACYCLINE @ SAME DOSE.
BODUNKOVA LE ET AL; ANTIBIOTIKI (MOSCOW) 20 (11): 1014-18 (1975)
14 hours
NORMAL HALF-LIFE: 15-17 HR. /FROM TABLE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1108
Methacycline, a tetracycline antibiotic, is a protein synthesis inhibitors, inhibiting the binding of aminoacyl-tRNA to the mRNA-ribosome complex. Methacycline inhibits cell growth by inhibiting translation. It binds to the 16S part of the 30S ribosomal subunit and prevents the amino-acyl tRNA from binding to the A site of the ribosome. The binding is reversible in nature. Tetracyclines also have been found to inhibit matrix metalloproteinases. This mechanism does not add to their antibiotic effects, but has led to extensive research on chemically modified tetracyclines or CMTs (like incyclinide) for the treatmet of rosacea, acne, and various types of neoplasms.
TETRACYCLINES ACT TO INHIBIT PROTEIN SYNTH &...BIND SPECIFICALLY TO 30 S RIBOSOMES. THEY APPEAR TO PREVENT ACCESS OF AMINOACYL TRNA TO MRNA-RIBOSOME COMPLEX. ONLY SMALL PORTION OF DRUG IS IRREVERSIBLY BOUND, & INHIBITORY EFFECTS...CAN BE REVERSED BY WASHING. /TETRACYCLINES/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1118


