



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0

1. D 20443
2. D 23129
3. D-20443
4. D-23129
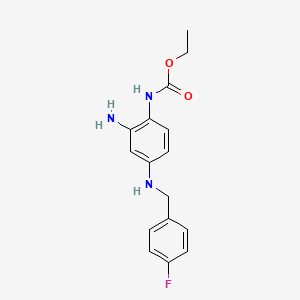
5. Ethyl N-(2-amino-4-(4-fluorobenzylamino)phenyl)carbamate Hydrochloride
6. Ezogabine
7. N-(2-amino-4-(4-fluorobenzylamino)phenyl)carbamic Acid Ethyl Ester
8. Potiga
1. 150812-12-7
2. Ezogabine
3. Potiga
4. Trobalt
5. D-23129
6. N-(2-amino-4-(4-fluorobenzylamino)phenyl)carbamic Acid Ethyl Ester
7. Ezogabine [usan]
8. Gke-841
9. Way-143841
10. Ethyl 2-amino-4-((p-fluorobenzyl)amino)carbanilate
11. Ethyl N-[2-amino-4-[(4-fluorophenyl)methylamino]phenyl]carbamate
12. Retigabine [inn]
13. Awd-21360
14. Awd21-360
15. Gw582892x
16. Gw-582892x
17. D 20443
18. N-(2-amino-4-(4-fluorobenzylamino)-phenyl) Carbamic Acid Ethyl Ester
19. Retigabine (1.0 Mg/ml In Acetonitrile)
20. Chembl41355
21. 12g01i6bbu
22. Chebi:68584
23. Ethyl (2-amino-4-(((4-fluorophenyl)methyl)amino)phenyl)carbamate
24. Retigabine (inn)
25. Ezogabine (usan)
26. Ethyl {2-amino-4-[(4-fluorobenzyl)amino]phenyl}carbamate
27. 2-amino-4-(4-fluorbenzylamino)-1-ethoxycarbonylaminobenzene
28. Ethyl [2-amino-4-[[(4-fluorophenyl)methyl]amino]phenyl]carbamate
29. Ethyl N-[2-azanyl-4-[(4-fluorophenyl)methylamino]phenyl]carbamate
30. [2-amino-4-(4-fluoro-benzylamino)-phenyl]-carbamic Acid Ethyl Ester
31. Retigabin
32. Potiva
33. D 23129
34. Gw 582892x
35. Way 143841
36. Unii-12g01i6bbu
37. Hsdb 8171
38. Gki 841
39. N03ax21
40. Fbx
41. Mfcd04307735
42. Potiga (tn)
43. Add-230001
44. Ke-0201
45. Ezogabine [mi]
46. Ezogabine [vandf]
47. Carbamic Acid, (2-amino-4-(((4-fluorophenyl)methyl)amino)phenyl)-, Ethyl Ester
48. Retigabine [mart.]
49. Retigabine [who-dd]
50. Schembl20835
51. Retigabine [ema Epar]
52. Retigabine, Analytical Standard
53. Gtpl2601
54. Dea No. 2779
55. Ezogabine [orange Book]
56. Retigabine, >=98% (hplc)
57. Retigabine-[d4] Dihydrochloride
58. Zinc16154
59. Dtxsid40164615
60. [2-amino-4-[[(4-fluorophenyl)methyl]amino]phenyl]-carbamate
61. Hms3748i09
62. Hms3885l20
63. Amy18466
64. Bcp04194
65. Ex-a2203
66. N-(2-amino-4-[fluorobenzylamino]-phenyl) Carbamic Acid
67. D-23129
68. Retigabine,d2312, D-23129
69. Bdbm50143558
70. S4733
71. Zinc00016154
72. Akos015895311
73. Ac-6908
74. Ccg-267503
75. Cs-0990
76. Db04953
77. Ncgc00346739-01
78. Ncgc00346739-05
79. Ncgc00346739-06
80. Aw21-360
81. Hy-15471
82. Bcp0726000089
83. Ft-0601527
84. D09569
85. 812r127
86. A809076
87. Q-101418
88. Q2146170
89. Ethyl 2-amino-4-(4-fluorobenzylamino)phenylcarbamate
90. Ethyl N-(2-amino-4-{[(4-fluorophenyl)methyl]amino}phenyl)carbamate
91. N-(2-amino-4-(4-fluorobenzylamino)-phenyl)carbamic Acid Ethyl Ester
92. N-(2-amino-4-(4-fluorobenzylamino)-phenyl)carbamic Acid Ethylester
93. N-[2-amino-4-[(4-fluorophenyl)methylamino]phenyl]carbamic Acid Ethyl Ester
94. Carbamic Acid, (2-amino-4-(((4-fluoro-phenyl)methyl)amino)phenyl)-ethyl Ester
95. Retigabine Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
96. Rtg
| Molecular Weight | 303.33 g/mol |
|---|---|
| Molecular Formula | C16H18FN3O2 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 303.13830499 g/mol |
| Monoisotopic Mass | 303.13830499 g/mol |
| Topological Polar Surface Area | 76.4 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 348 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Potiga |
| PubMed Health | Ezogabine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | The chemical name of ezogabine is N-[2-amino-4-(4-fluorobenzylamino)-phenyl] carbamic acid ethyl ester, and it has the following structure:The empirical formula is C16H18FN3O2, representing a molecular weight of 303.3. Ezogabine is a white to slightl... |
| Active Ingredient | Ezogabine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg; 300mg; 50mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 2 | |
|---|---|
| Drug Name | Potiga |
| PubMed Health | Ezogabine (By mouth) |
| Drug Classes | Anticonvulsant |
| Drug Label | The chemical name of ezogabine is N-[2-amino-4-(4-fluorobenzylamino)-phenyl] carbamic acid ethyl ester, and it has the following structure:The empirical formula is C16H18FN3O2, representing a molecular weight of 303.3. Ezogabine is a white to slightl... |
| Active Ingredient | Ezogabine |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 400mg; 300mg; 50mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Anticonvulsants; Membrane Transport Modulators
National Library of Medicine's Medical Subject Headings. Ezogabine. Online file (MeSH, 2014). Available from, as of January 30, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Potiga is indicated as adjunctive treatment of partial-onset seizures in patients aged 18 years and older who have responded inadequately to several alternative treatments and for whom the benefits outweigh the risk of retinal abnormalities and potential decline in visual acuity. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for POTIGA (ezogabine) tablet, film coated (September 2013). Available from, as of February 12, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0c60979b-489d-4e7b-8893-468ae00c44bb
/BOXED WARNING/ WARNING: RETINAL ABNORMALITIES AND POTENTIAL VISION LOSS. Potiga can cause retinal abnormalities with funduscopic features similar to those seen in retinal pigment dystrophies, which are known to result in damage to the photoreceptors and vision loss. Some patients with retinal abnormalities have been found to have abnormal visual acuity. It is not possible to determine whether Potiga caused this decreased visual acuity, as baseline assessments are not available for these patients. Approximately one third of the patients who had eye examinations performed after approximately 4 years of treatment were found to have retinal pigmentary abnormalities. An earlier onset cannot be ruled out, and it is possible that retinal abnormalities were present earlier in the course of exposure to Potiga. The rate of progression of retinal abnormalities and their reversibility are unknown. Potiga should only be used in patients who have responded inadequately to several alternative treatments and for whom the benefits outweigh the potential risk of vision loss. Patients who fail to show substantial clinical benefit after adequate titration should be discontinued from Potiga. All patients taking Potiga should have baseline and periodic (every 6 months) systematic visual monitoring by an ophthalmic professional. Testing should include visual acuity and dilated fundus photography. Additional testing may include fluorescein angiograms (FA), ocular coherence tomography (OCT), perimetry, and electroretinograms (ERG). If retinal pigmentary abnormalities or vision changes are detected, Potiga should be discontinued unless no other suitable treatment options are available and the benefits of treatment outweigh the potential risk of vision loss.
US Natl Inst Health; DailyMed. Current Medication Information for POTIGA (ezogabine) tablet, film coated (September 2013). Available from, as of February 12, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0c60979b-489d-4e7b-8893-468ae00c44bb
Skin discoloration and eye abnormalities characterized by pigment changes in the retina have been reported in patients receiving ezogabine. In the cases reported to date, the skin discoloration appeared as blue pigmentation, predominantly on or around the lips or in the nail beds of the fingers or toes; however, more widespread involvement of the face and legs has also been reported. In addition, scleral and conjunctival discoloration (on the white of the eye and inside the eyelids) has been observed. The blue skin discoloration generally occurred following 4 years of treatment with ezogabine, but has been observed sooner in some patients. In some cases, retinal abnormalities were observed in the absence of skin discoloration. It is not yet known if the retinal and skin changes are reversible. All patients receiving ezogabine should have a baseline or initial eye examination followed by periodic eye examinations that should include visual acuity testing and dilated fundus photography, and may include fluorescein angiograms (FA), ocular coherence tomography (OCT), perimetry, and electroretinograms (ERG). If ophthalmologic changes are observed, ezogabine should be discontinued unless no other treatment options are available. If a patient develops skin discoloration, serious consideration should be given to changing to an alternative anticonvulsant agent.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Suicidal behavior and ideation have been reported in patients receiving anticonvulsants, including ezogabine. The US Food and Drug Administration (FDA) has alerted healthcare professionals about an increased risk of suicidality (suicidal behavior or ideation) observed in an analysis of studies using various anticonvulsants compared with placebo. The analysis of suicidality reports from placebo-controlled studies involving 11 anticonvulsants (i.e., carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, zonisamide) in patients with epilepsy, psychiatric disorders (e.g., bipolar disorder, depression, anxiety), and other conditions (e.g., migraine, neuropathic pain) found that patients receiving anticonvulsants had approximately twice the risk of suicidal behavior or ideation (0.43%) compared with patients receiving placebo (0.24%). This increased suicidality risk was observed as early as one week after beginning therapy and continued through 24 weeks. Although patients treated with an anticonvulsant for epilepsy, psychiatric disorders, and other conditions were all found to have an increased suicidality risk compared with those receiving placebo, the relative suicidality risk was higher for patients with epilepsy compared with those receiving anticonvulsants for other conditions. Based on the current analysis of the available data, the FDA recommends that clinicians inform patients, their families, and caregivers of the potential for an increased risk of suicidality with anticonvulsant therapy and that all patients currently receiving or beginning therapy with any anticonvulsant be closely monitored for notable changes that may indicate the emergence or worsening of suicidal thoughts or behavior or depression. Symptoms such as anxiety, agitation, hostility, insomnia, and mania may be precursors to emerging suicidality. Clinicians who prescribe ezogabine or any other anticonvulsant should balance the risk of suicidality with the clinical need for the drug and the risk associated with untreated illness. Epilepsy and many other illnesses for which anticonvulsants are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. If suicidal thoughts or behaviors emerge during anticonvulsant therapy, the clinician should consider whether these symptoms may be related to the illness being treated.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
As with all anticonvulsant agents, ezogabine therapy should be withdrawn gradually whenever possible to minimize the risk of increased seizure frequency. When discontinuing therapy, dosage should be reduced over a period of at least 3 weeks unless safety concerns require more rapid withdrawal.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
For more Drug Warnings (Complete) data for Ezogabine (19 total), please visit the HSDB record page.
Adjuvant treatment of partial-onset seizures.
FDA Label
Trobalt is indicated as adjunctive treatment of drug-resistant partial-onset seizures with or without secondary generalisation in patients aged 18 years or older with epilepsy, where other appropriate drug combinations have proved inadequate or have not been tolerated.
As compared to other antiepileptic agents, ezogabine is unique in that it selectively activates potassium ion channels Kv 7.2-Kv7.5 and not cardiac Kv 7.1, thereby avoiding cardiac side effects. The antiepileptics, as a drug class, are routinely used in the treatment of a number of disease states in addition to epilepsy. Ezogabine is highly efficacious in a broad-spectrum of in vivo epilepsy and seizure models. A comparison of antiepileptic form activity of ezogabine with that of conventional anticonvulsants in in vitro models suggests that retigabine is especially likely to be useful in the treatment of pharmacoresistant epilepsy. Retigabine clearly attenuates pain-like behaviors in various animal models of neuropathic pain; it may also prove to be useful in treatment of clinical anxiety disorders. Clinical data obtained thus far indicate that retigabine is well tolerated in humans when titrated up to its therapeutic dose range. No tolerance, drug dependence, or withdrawal liability has been reported. Thus, retigabine may prove to be useful in the treatment of a diverse range of disease states in which neuronal hyperexcitability is a common underlying factor.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Membrane Transport Modulators
Agents that affect ION PUMPS; ION CHANNELS; ABC TRANSPORTERS; and other MEMBRANE TRANSPORT PROTEINS. (See all compounds classified as Membrane Transport Modulators.)
N03AX21
N03AX21
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AX - Other antiepileptics
N03AX21 - Retigabine
Absorption
Rapidly absorbed and distributed, with an absolute oral bioavailability of 60%. Pharmacokinetics of ezogabine suggest first-order kinetics. Tmax, single oral dose = 30-120 minutes; Time to steady state = 3 days
Route of Elimination
Urine (85%, 36% of total dose as unchanged drug, 18% of total dose as NAMR) and feces (14%, 3% of total dose as unchanged drug)
Volume of Distribution
8.7 L/kg
Clearance
0.58 - 0.76 L/hkg. Clearance may differ between ethnic groups with Black Americans having 20% lower clearance than Caucasian Americans.
In all tested species, retigabine was rapidly absorbed from the gastro-intestinal tract following single PO administration (< 2 hr). Dietary dosing in rodents expectedly resulted in substantially lower Cmax values obtained several hours after treatment.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Trobalt p.13 (2011). Available from, as of February 12, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001245/WC500104839.pdf
In rat and dogs studies evaluating the excretion of retigabine and the N-acetyl metabolite of ezogabine (NAMR) showed that retigabine and metabolites were excreted in both faeces and urine, with an approximate 2:1 faeces:urine split of the eliminated dose. Studies with bile duct cannulated rats showed that a major part of the retigabine-related radioactivity in faeces originated from the bile. NAMR was excreted to a greater extent in the urine, i.e., approximately one-half to two-third of the dose. Elimination patterns were similar following both PO and IV dosing, and the elimination of drug via the faeces following IV dosing was in accordance with significant biliary excretion.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Trobalt p.14 (2011). Available from, as of February 12, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001245/WC500104839.pdf
Data from in vitro studies indicate that ezogabine and the N-acetyl metabolite of ezogabine (NAMR) are approximately 80% and 45% bound to plasma protein, respectively. Clinically significant interactions with other drugs through displacement from proteins are not anticipated. The steady-state volume of distribution of ezogabine is 2 to 3 L/kg following intravenous dosing, suggesting that ezogabine is well distributed in the body.
US Natl Inst Health; DailyMed. Current Medication Information for POTIGA (ezogabine) tablet, film coated (September 2013). Available from, as of February 12, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0c60979b-489d-4e7b-8893-468ae00c44bb
After both single and multiple oral doses, ezogabine is rapidly absorbed with median time to maximum plasma concentration (Tmax) values generally between 0.5 and 2 hours. Absolute oral bioavailability of ezogabine relative to an intravenous dose of ezogabine is approximately 60%. High-fat food does not affect the extent to which ezogabine is absorbed based on plasma AUC values, but it increases peak concentration (Cmax) by approximately 38% and delays Tmax by 0.75 hour.
US Natl Inst Health; DailyMed. Current Medication Information for POTIGA (ezogabine) tablet, film coated (September 2013). Available from, as of February 12, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0c60979b-489d-4e7b-8893-468ae00c44bb
For more Absorption, Distribution and Excretion (Complete) data for Ezogabine (9 total), please visit the HSDB record page.
Ezogabine is metabolized exclusively via phase II hepatic N-glucurodination and acetylation. N-glucurodination is the major metabolic pathway of the two and form two major N-glucuronide metabolites. The enzymes involved are UGT1A1, 1A9, 1A4, and 1A3. However, the product of the N-acetyl pathway is a weak, active metabolite referred to as NAMR. The enzyme that is involved in the N-acetyl pathway is called N-acetyltransferase 2. The pharmacokinetics of NAMR and ezogabine are similar. The cytochrome P450 enzyme system is not involved with the metabolism of ezogabine.
The primary routes of retigabine metabolism were dominated by phase II processes involving hydrolysis/N-acetylation to form the N-acetyl metabolite of ezogabine (NAMR) and N-glucuronidation of retigabine and NAMR. There was no evidence of direct oxidative metabolism of retigabine in any species. There was no evidence of NAMR formation in the dog and little NAMR formation in the Cynomolgus monkey. All evaluated species formed N-glucuronides of retigabine. N-glucoside metabolites of RTG were also observed in dog and humans.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Trobalt p.14 (2011). Available from, as of February 12, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001245/WC500104839.pdf
Ezogabine is extensively metabolized primarily via glucuronidation and acetylation in humans. A substantial fraction of the ezogabine dose is converted to inactive N-glucuronides, the predominant circulating metabolites in humans. Ezogabine is also metabolized to the N-acetyl metabolite of ezogabine (NAMR) that is also subsequently glucuronidated. NAMR has antiepileptic activity, but it is less potent than ezogabine in animal seizure models. Additional minor metabolites of ezogabine are an N-glucoside of ezogabine and a cyclized metabolite believed to be formed from NAMR. In vitro studies using human biomaterials showed that the N-acetylation of ezogabine was primarily carried out by NAT2, while glucuronidation was primarily carried out by UGT1A4, with contributions by UGT1A1, UGT1A3, and UGT1A9. In vitro studies showed no evidence of oxidative metabolism of ezogabine or NAMR by cytochrome P450 enzymes. Coadministration of ezogabine with medications that are inhibitors or inducers of cytochrome P450 enzymes is therefore unlikely to affect the pharmacokinetics of ezogabine or NAMR.
US Natl Inst Health; DailyMed. Current Medication Information for POTIGA (ezogabine) tablet, film coated (September 2013). Available from, as of February 12, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0c60979b-489d-4e7b-8893-468ae00c44bb
Retigabine has known human metabolites that include retigabine N2-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Terminal half-life = 7.5 hours
A high variability was generally observed in the elimination half-life values of retigabine. The half-life was in the range of 1.4-9 hr in rats, 4-22 hr in rabbits, 0.9-20 hr in dogs, compared to 6-10 hr in humans. Thus, rats were generally having lower values for half-life than rabbits, dogs and humans.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Trobalt p.13 (2011). Available from, as of February 12, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001245/WC500104839.pdf
In pregnant rats, the tissue distribution pattern was similar in dams and fetuses but the tissue exposure in fetal tissue was lower and more uniform with no high concentration organs as seen in the dams. The tissue elimination half lives in dams were between 4-18 hours and in fetuses between 6-12 hours.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Trobalt p.13-4 (2011). Available from, as of February 12, 2014: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001245/WC500104839.pdf
Ezogabine has a novel mechanism of action that involves opening of neuronal Kv7.2-7.5 (formerly KCNQ2-5) voltage activated potassium channels. These channels (primarily Kv7.2/7.3) enable generation of the M-current, a sub-threshold potassium current that serves to stabilize the membrane potential and control neuronal excitability. In addition to acting on potassium ion channels, retigabine also affects GABA neurotransmission in the GABA-A receptor, which is a key inhibitory receptor in the central nervous system and is implicated in epilepsy. Malfunctioning of the GABA-A receptor leads to hyperexcitability in the brain, which causes seizures, making this receptor an important target for antiepileptic therapeutics. Apart from increasing the concentration of GABA in the brain (by either enhancing GABA synthesis or blocking GABA metabolism), retigabine allosterically potentiates GABA-induced current in rat cortical neurons in a concentration-dependent manner. Numerous studies have demonstrated that retigabine is effective in a broad spectrum of in vivo epilepsy and seizure models.
The pharmacologic profile of retigabine (RTG (international nonproprietary name); ezogabine, EZG (U.S. adopted name)), is different from all currently approved antiepileptic drugs (AEDs). Its primary mechanism of action (MoA) as a positive allosteric modulator of KCNQ2-5 (K(v) 7.2-7.5) ion channels defines RTG/EZG as the first neuronal potassium (K(+)) channel opener for the treatment of epilepsy. KCNQ2-5 channels are predominantly expressed in neurons and are important determinants of cellular excitability, as indicated by the occurrence of human genetic mutations in KCNQ channels that underlie inheritable disorders including, in the case of KCNQ2/3, the syndrome of benign familial neonatal convulsions. In vitro pharmacologic studies demonstrate that the most potent action of RTG/EZG is at KCNQ2-5 channels, particularly heteromeric KCNQ2/3. Furthermore, mutagenesis and modeling studies have pinpointed the RTG/EZG binding site to a hydrophobic pocket near the channel gate, indicating how RTG/EZG can stabilize the open form of KCNQ2-5 channels; the absence of this site in KCNQ1 also provides a clear explanation for the inbuilt selectivity RTG/EZG has for potassium channels other than the KCNQ cardiac channel. KCNQ channels are active at the normal cell resting membrane potential (RMP) and contribute a continual hyperpolarizing influence that stabilizes cellular excitability. The MoA of RTG/EZG increases the number of KCNQ channels that are open at rest and also primes the cell to retort with a larger, more rapid, and more prolonged response to membrane depolarization or increased neuronal excitability. In this way, RTG/EZG amplifies this natural inhibitory force in the brain, acting like a brake to prevent the high levels of neuronal action potential burst firing (epileptiform activity) that may accompany sustained depolarizations associated with the initiation and propagation of seizures. This action to restore physiologic levels of neuronal activity is thought to underlie the efficacy of RTG/EZG as an anticonvulsant in a broad spectrum of preclinical seizure models and in placebo-controlled trials in patients with partial epilepsy. ...
PMID:22220513 Gunthorpe MJ et al; Epilepsia 53 (3): 412-24 (2012)
The mechanism by which ezogabine exerts its therapeutic effects has not been fully elucidated. In vitro studies indicate that ezogabine enhances transmembrane potassium currents mediated by the KCNQ (Kv7.2 to 7.5) family of ion channels. By activating KCNQ channels, ezogabine is thought to stabilize the resting membrane potential and reduce brain excitability. In vitro studies suggest that ezogabine may also exert therapeutic effects through augmentation of GABA-mediated currents.
US Natl Inst Health; DailyMed. Current Medication Information for POTIGA (ezogabine) tablet, film coated (September 2013). Available from, as of February 12, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0c60979b-489d-4e7b-8893-468ae00c44bb








