Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
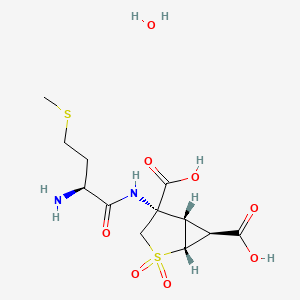

1. Ly2140023 Monohydrate
1. Ly2140023 Monohydrate
2. 956385-05-0
3. Ly-2140023 Monohydrate
4. Pomaglumetad Metionilo
5. We665jb15r
6. (1r,4s,5s,6s)-4-(l-methionylamino)-2-thiabicyclo(3.1.0)hexane-4,6-dicarboxylic Acid 2,2-dioxide Monohydrate
7. Dtxsid00212944
8. 4-(((2s)-2-amino-4-(methylthio)butanoyl)amino)-2-thiabicyclo(3.1.0)hexane-4,6-dicarboxylic Acid 2,2-dioxide Hydrate, 1r,4s,5s,6s
9. Pomaglumetadum Methionilum
10. Refchem:175219
11. Dtxcid30135435
12. 2-thiabicyclo(3.1.0)hexane-4,6-dicarboxylic Acid, 4-(((2s)-2-amino-4-(methylthio)-1-oxobutyl)amino)-, 2,2-dioxide, Hydrate (1:1), (1r,4s,5s,6s)-
13. 691-416-7
14. Pomaglumetad Methionil [usan]
15. Pomaglumetad Methionil (usan)
16. (1r,4s,5s,6s)-4-((s)-2-amino-4-(methylthio)butanamido)-2-thiabicyclo[3.1.0]hexane-4,6-dicarboxylic Acid 2,2-dioxide Hydrate
17. (1r,4s,5s,6s)-4-[[(2s)-2-amino-4-methylsulfanylbutanoyl]amino]-2,2-dioxo-2lambda6-thiabicyclo[3.1.0]hexane-4,6-dicarboxylic Acid;hydrate
18. Unii-we665jb15r
19. Pomaglumetad Methionil Hydrate
20. Orb1708826
21. Schembl1100180
22. Chembl2105707
23. Dtxsid10241907
24. Pomaglumetad Methionil Monohydrate
25. Akos040735205
26. Hy-105040
27. Cs-0024754
28. D09949
29. Q27292586
30. (1r,4s,5s,6s)-4-(2s-4-methylthio-2-aminobutanonyl)amino-2,2-dioxo-2lambda6-thia- Bicyclo(3.1.0)hexane-4,6-dicarboxylic Acid Monohydrate
31. (1r,4s,5s,6s)-4-(l-methionylamino)-2,2-dioxo-2gamma6-thiabicyclo[3.1.0]hexane-4,6-dicarboxylic Acid-water (1:1)
32. (1r,4s,5s,6s)-4-(l-methionylamino)-2,2-dioxo-2lambda~6~-thiabicyclo[3.1.0]hexane-4,6-dicarboxylic Acid--water (1/1)
33. (1r,4s,5s,6s)-4-[[(2s)-2-amino-4-methylsulfanylbutanoyl]amino]-2,2-dioxo-2
34. E6-thiabicyclo[3.1.0]hexane-4,6-dicarboxylic Acid;hydrate
35. 2-thiabicyclo(3.1.0)hexane-4,6-dicarboxylic Acid, 4-(((2s)-2-amino-4-(methylthio)-1- Oxobutyl)amino)-, 2,2-dioxide, Hydrate (1:1), (1r,4s,5s,6s)-
| Molecular Weight | 384.4 g/mol |
|---|---|
| Molecular Formula | C12H20N2O8S2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 199 |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 644 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Investigated for use/treatment in psychosis and schizophrenia and schizoaffective disorders.
LY2140023 is an antipsychotic agent that is a metabotropic glutamate 2/3 receptor agonist. This agent has a new mechanism of action that is efficacious in treating schizophrenia and potentially other neuropsychiatric conditions. Once absorbed, LY2140023 is efficiently hydrolyzed to produce the active mGlu2/3 receptor agonist LY404039. LY404039 and other mGlu2/3 agonists do not directly interact with dopamine or serotonin (5-HT2A) receptors. However, 'functional' 5-HT2A receptor antagonism in the prefrontal cortex may represent a common mechanism shared by clinically effective atypical antipsychotics and mGlu2/3 receptor agonists, and may contribute to the antipsychotic actions of LY2140023.
ABOUT THIS PAGE

