



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
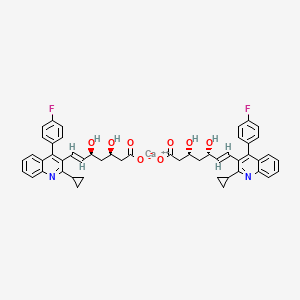

1. (e,3r,5s)-7-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-3,5-dihydroxyhept-6-enoic Acid
2. Itavastatin
3. Itavastatin Calcium
4. Nisvastatin
5. Nk 104
6. Nk-104
7. P 872441
8. P-872441
9. Pitavastatin
10. Pitavastatin Calcium
11. Pitavastatin Lactone
1. Pitavastatin Calcium
2. 147526-32-7
3. Livalo
4. Nisvastatin
5. Nk-104
6. Itavastatin Calcium
7. Pitavastatin Calcium Salt
8. Calcium (3r,5s,e)-7-(2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-3,5-dihydroxyhept-6-enoate
9. Nk 104
10. Iyd54xeg3w
11. Chebi:71258
12. Calcium;(e,3r,5s)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxyhept-6-enoate
13. P-872441
14. Pitavastatin Calcium (jan)
15. Pitavastatin Calcium [jan]
16. Alipza
17. Flovas
18. Livazo
19. Unii-iyd54xeg3w
20. Nk 104 (acid)
21. Redevant
22. Ccris 8652
23. Livalo (tn)
24. Schembl22720
25. Bis((3r,5s,6e)-7-(2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl)-3,5-dihydroxy-6-heptenoate), Monocalcium Salt
26. Chembl1237061
27. Dtxsid4046448
28. Chebi:94569
29. Pitavastatin Hemicalcium;nk-104
30. Nks-104
31. Act02718
32. Mfcd01937979
33. Pitavastatin Calcium [mart.]
34. Pitavastatin Calcium [who-dd]
35. Akos015900407
36. Pitavastatin Calcium Salt [mi]
37. Am84441
38. Ks-1220
39. (+)-monocalciumbis{(3r,5s,6e)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5-dihydroxy-6-hepten
40. 111ge002
41. 6-heptenoic Acid, 7-(2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl)-3,5-dihydroxy-, Calcium Salt (2:1), (s-(r*,s*-(e)))-
42. Pitavastatin Calcium [orange Book]
43. D01862
44. Q-201590
45. Q27139472
46. (+)-monocalciumbis[(3r,5s,6e)-7-[2-cyclopropyl-4(4-fluorophenyl)-3-quinolyl]3,5-dihydroxy-6-hepteno Ate]
47. (3r,5s,6e)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinyl]-3,5-dihydroxy-6-heptenoic Acid Hemicalcium Salt
48. Bis((3r,5s,6e)-7-(2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl)-3,5-dihydroxy-6-heptenoate) Monocalcium Salt
49. Calcium (3r,5s,e)-7-(2-cyclopropyl-4-(4-fluorophenyl)-quinolin-3-yl)-3,5-dihydroxyhept-6-enoate
50. Calcium Bis{(3r,5s,6e)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxyhept-6-enoate}
| Molecular Weight | 881.0 g/mol |
|---|---|
| Molecular Formula | C50H46CaF2N2O8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 14 |
| Exact Mass | 880.2848136 g/mol |
| Monoisotopic Mass | 880.2848136 g/mol |
| Topological Polar Surface Area | 187 Ų |
| Heavy Atom Count | 63 |
| Formal Charge | 0 |
| Complexity | 626 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Livalo |
| PubMed Health | Pitavastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Active Ingredient | Pitavastatin calcium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 4mg base; eq 2mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Kowa |
| 2 of 2 | |
|---|---|
| Drug Name | Livalo |
| PubMed Health | Pitavastatin (By mouth) |
| Drug Classes | Antihyperlipidemic |
| Active Ingredient | Pitavastatin calcium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 4mg base; eq 2mg base; eq 1mg base |
| Market Status | Prescription |
| Company | Kowa |
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Compounds that inhibit HYDROXYMETHYLGLUTARYL COA REDUCTASES. They have been shown to directly lower CHOLESTEROL synthesis. (See all compounds classified as Hydroxymethylglutaryl-CoA Reductase Inhibitors.)






