
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
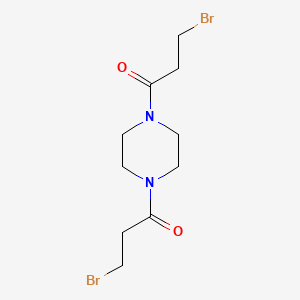

1. Vercyte
1. 54-91-1
2. Vercyte
3. Amedel
4. 1,1'-(piperazine-1,4-diyl)bis(3-bromopropan-1-one)
5. 1,4-bis(3-bromopropionyl)piperazine
6. Pipobromanum
7. A-8103
8. Piperazine, 1,4-bis(3-bromo-1-oxopropyl)-
9. Nsc-25154
10. N,n-bis-(3-bromopropionyl)-piperazine
11. A 1803
12. Piperazine, 1,4-bis(3-bromopropionyl)-
13. 3-bromo-1-[4-(3-bromopropanoyl)piperazin-1-yl]propan-1-one
14. 1,4-bis(3-bromopropanoyl)piperazine
15. N,n'-bis(3-bromopropionyl)piperazine
16. Nsc25154
17. 6q99rdt97r
18. Chebi:8242
19. Ncgc00095048-01
20. Dsstox_cid_3485
21. Dsstox_rid_77048
22. Dsstox_gsid_23485
23. Pipobromanum [inn-latin]
24. Cas-54-91-1
25. Ccris 2753
26. Hsdb 3249
27. Sr-05000001858
28. Vercyte (tn)
29. Nsc 25154
30. Brn 0749866
31. Unii-6q99rdt97r
32. Pipobroman (jan/usan/inn)
33. Pipobroman [usan:usp:inn]
34. Ai3-50113
35. Spectrum_001727
36. Pipobroman [mi]
37. Pipobroman [inn]
38. Pipobroman [jan]
39. Spectrum2_000912
40. Spectrum3_001047
41. Spectrum5_001135
42. Pipobroman [hsdb]
43. Pipobroman [usan]
44. 4-chlorophenylphenylsulfone
45. Pipobroman [vandf]
46. Pipobroman [mart.]
47. Schembl4889
48. Chembl1585
49. Pipobroman [who-dd]
50. Bspbio_002574
51. Kbioss_002207
52. Divk1c_000799
53. Spectrum1503393
54. Spbio_000784
55. Gtpl7271
56. Dtxsid7023485
57. Pipobroman [orange Book]
58. Hms502h21
59. Kbio1_000799
60. Kbio2_002207
61. Kbio2_004775
62. Kbio2_007343
63. Kbio3_001794
64. Ninds_000799
65. Hms1922c10
66. Hms2093e11
67. Pharmakon1600-01503393
68. Wln: T6n Dntj Av2e Dv2e
69. Bcp31516
70. Zinc1530753
71. Tox21_111402
72. Ccg-39748
73. Mfcd00866372
74. Nsc758461
75. Piperazine,4-bis(3-bromopropionyl)-
76. S4447
77. 1, 4-bis(3-bromopropionyl)piperazine
78. Akos016010321
79. Tox21_111402_1
80. Cs-5085
81. Db00236
82. Ds-4654
83. Nsc-758461
84. 1,4-bis(3-bromopropanoyl)piperazine #
85. Idi1_000799
86. Ncgc00095048-02
87. Ncgc00095048-03
88. Ncgc00095048-04
89. Ncgc00095048-06
90. Hy-16398
91. Nci60_002008
92. Piperazine,4-bis(3-bromo-1-oxopropyl)-
93. A 8103
94. C07362
95. D00467
96. W18797
97. Ab00052352-02
98. Ab00052352_03
99. 1,1'-piperazine-1,4-diylbis(3-bromopropan-1-one)
100. Sr-05000001858-1
101. Sr-05000001858-2
102. Q15366704
103. Vercyte;nsc-25154;1,4-bis(3-bromo-1-oxopropyl)piperazine
| Molecular Weight | 356.05 g/mol |
|---|---|
| Molecular Formula | C10H16Br2N2O2 |
| XLogP3 | 0.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 355.95580 g/mol |
| Monoisotopic Mass | 353.95785 g/mol |
| Topological Polar Surface Area | 40.6 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 227 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antineoplastic Agents, Alkylating
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...ANTINEOPLASTIC DRUG OF ALKYLATING TYPE. @ PRESENT ITS USE IS MAINLY LIMITED TO TREATMENT OF POLYCYTHEMIA VERA & CHRONIC GRANULOCYTIC LEUKEMIA. EVEN IN THESE DISORDERS...PIPOBROMAN IS GENERALLY NOT AS EFFECTIVE AS OLDER MODES OF TREATMENT.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1083
...PIPOBROMAN IS HELD IN RESERVE FOR USE IN PATIENTS THAT HAVE BECOME REFRACTORY TO X-IRRADIATION & BUSULFAN IN CASE OF LEUKEMIA & PHLEBOTOMY & RADIOPHOSPHATE IN CASE OF POLYCYTHEMIA VERA.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1083
Pipobroman is indicated mainly in the treatment of polycythemia vera. In the limited number of studies reported, there seems to be no difference in the therapeutic response between patients who had been under prior treatment with other drugs and those who had not. There are insufficient data available to provide a comparison of the effectiveness of pipobroman with conventional forms of treatment (radioactive phosphorus, phlebotomy, or other alkylating agents); however, it may be effective in patients with polycythemia vera who are refractory to these forms of therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 725
Although pipobroman has produced remission in chronic myelocytic (granulocytic) leukemia, the number of reported cases is too few to permit evaluation of the drug for this condition and its use is therefore reserved for patients whose disease is resistant to other therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 725
PATIENTS IN WHOM BONE MARROW FUNCTION IS STILL DEPRESSED FROM PREVIOUS IRRADIATION OR OTHER CYTOTOXIC CHEMOTHERAPY SHOULD NOT RECEIVE PIPOBROMAN. CLINICAL EXPERIENCE WITH THIS DRUG IS INSUFFICIENT TO RECOMMEND ITS USE IN CHILDREN UNDER 15 YR OF AGE.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1116
PIPOBROMAN...SHOULD NOT BE ADMIN DURING PREGNANCY, SINCE THERE IS NO INFORMATION ON ITS POTENTIAL TERATOGENICITY.
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 1116
Rash and adverse GI effects such as nausea, vomiting, abdominal cramping, diarrhea, and anorexia may occur. These adverse effects are usually transient, but may persist and necessitate cessation of therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 726
Pipobroman should not be administered to patients with bone marrow depression resulting from radiation therapy or cytotoxic chemotherapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 726
For the treatment of polycythaemia vera and refractory chronic myeloid leukaemia.
Pipobroman is an antineoplastic agent. Specifically it is a piperazine derivative with a chemical structure close to that of many DNA alkylating agents. Pipobroman has well documented clinical activity against polycythemia vera and essential thrombocythemia.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AX - Other alkylating agents
L01AX02 - Pipobroman
Absorption
Well absorbed from the GI tract.
The drug is well absorbed following oral administration. Studies of its distribution and fate in man have not been reported.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 725
The mechanism of action is uncertain but pipobroman is thought to alkylate DNA leading to disruption of DNA synthesis and eventual cell death.
Pipobroman has been classified as a polyfunctional alkylating agent, but its precise mechanism of action is not known.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 95. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1995 (Plus Supplements 1995)., p. 725
ABOUT THIS PAGE
17
PharmaCompass offers a list of Pipobroman API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pipobroman manufacturer or Pipobroman supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pipobroman manufacturer or Pipobroman supplier.
PharmaCompass also assists you with knowing the Pipobroman API Price utilized in the formulation of products. Pipobroman API Price is not always fixed or binding as the Pipobroman Price is obtained through a variety of data sources. The Pipobroman Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pipobroman manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pipobroman, including repackagers and relabelers. The FDA regulates Pipobroman manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pipobroman API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Pipobroman supplier is an individual or a company that provides Pipobroman active pharmaceutical ingredient (API) or Pipobroman finished formulations upon request. The Pipobroman suppliers may include Pipobroman API manufacturers, exporters, distributors and traders.
Pipobroman Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pipobroman GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pipobroman GMP manufacturer or Pipobroman GMP API supplier for your needs.
A Pipobroman CoA (Certificate of Analysis) is a formal document that attests to Pipobroman's compliance with Pipobroman specifications and serves as a tool for batch-level quality control.
Pipobroman CoA mostly includes findings from lab analyses of a specific batch. For each Pipobroman CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pipobroman may be tested according to a variety of international standards, such as European Pharmacopoeia (Pipobroman EP), Pipobroman JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pipobroman USP).
