



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
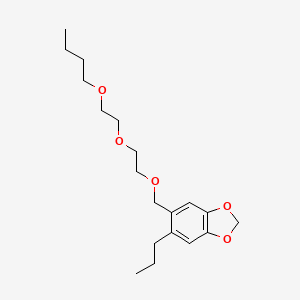

1. Butoxide, Piperonyl
1. 51-03-6
2. Butacide
3. Piperonylbutoxide
4. Butocide
5. Ethanol Butoxide
6. Pyrenone 606
7. 5-((2-(2-butoxyethoxy)ethoxy)methyl)-6-propylbenzo[d][1,3]dioxole
8. Butyl Carbitol 6-propylpiperonyl Ether
9. 6-propylpiperonyl Butyl Diethylene Glycol Ether
10. Butoxide (synergist)
11. 2-(2-butoxyethoxy)ethyl 6-propylpiperonyl Ether
12. Nci-c02813
13. Fmc 5273
14. Nia 5273
15. 6-(propylpiperonyl)butylcarbityl Ether
16. Ent 14,250
17. Piperonyl Butoxide [ban]
18. Nsc 8401
19. 1,3-benzodioxole, 5-[[2-(2-butoxyethoxy)ethoxy]methyl]-6-propyl-
20. Piperonyl Butoxide In Solvent
21. (butylcarbityl)(6-propylpiperonyl)ether
22. 1,3-benzodioxole, 5-((2-(2-butoxyethoxy)ethoxy)methyl)-6-propyl-
23. Nsc-8401
24. Ent-14250
25. Lwk91tu9ah
26. 3,4-methylenedioxy-6-propylbenzyl N-butyl Diethyleneglycol Ether
27. 5-[2-(2-butoxyethoxy)ethoxymethyl]-6-propyl-1,3-benzodioxole
28. Piperonyl Butoxide Technical
29. Alpha-(2-(2-n-butoxyethoxy)-ethoxy)-4,5-methylenedioxy-2-propyltoluene
30. Dtxsid1021166
31. 3,4-methylendioxy-6-propylbenzyl-n-butyl-diaethylenglykolaether
32. Chebi:32687
33. 5-((2-(2-butoxyethoxy)ethoxy)methyl)-6-propyl-1,3-benzodioxole
34. 5-propyl-4-(2,5,8-trioxa-dodecyl)-1,3-benzodioxole
35. Butylcarbityl (6-propylpiperonyl) Ether
36. Ncgc00090874-02
37. Ncgc00090874-04
38. 5-{[2-(2-butoxyethoxy)ethoxy]methyl}-6-propyl-1,3-benzodioxole
39. (3,4-methylenedioxy-6-propylbenzyl) (butyl) Diethylene Glycol Ether
40. Dsstox_cid_1166
41. Piperonyl Butoxide (ban)
42. Dsstox_rid_75988
43. Dsstox_gsid_21166
44. Butoxide, Piperonyl
45. Caswell No. 670
46. Alleviate
47. 5-(2-(2-butoxyethoxy)ethoxymethyl)-6-propyl-1,3-benzodioxole
48. 5-[[2-(2-butoxyethoxy)ethoxy]methyl]-6-propyl-1,3-benzodioxole
49. 5-{[(2-{[2-(butyloxy)ethyl]oxy}ethyl)oxy]methyl}-6-propyl-1,3-benzodioxole
50. Alpha-[2-(2-butoxyethoxy)ethoxy]-4,5-(methylenedioxy)-2-propyltoluene
51. Piperonyl Butoxyde
52. Cas-51-03-6
53. Piperonyl Butoxide, Technical
54. Ccris 522
55. Hsdb 1755
56. Piperonyl Butoxyde [iso-french]
57. Einecs 200-076-7
58. Unii-lwk91tu9ah
59. Epa Pesticide Chemical Code 067501
60. Brn 0288063
61. 6-(propylpiperonyl)-butyl Carbityl Ether
62. Butyl-carbityl (6-propylpiperonyl) Ether
63. Pyrenon
64. Ai3-14250
65. Piperonyl Butoxide [bsi:iso]
66. .alpha.(2-(2-butoxyethoxy)ethoxy)-4,5-methylenedioxy-2-propyltoluene
67. .alpha.-(2-(2-n-butoxyethoxy)-ethoxy)-4,5-methylenedioxy-2-propyltoluene
68. .alpha.-[2-(2-n-butoxyethoxy)-ethoxy]-4,5-methylenedioxy-2-propyltoluene
69. .alpha.[2-(2-butoxyethoxy)ethoxy]-4,5-methylenedioxy-2-propyltoluene
70. Piperonyl-butoxide
71. Rid Mousse (tn)
72. Scourge (salt/mix)
73. Para Pio (tn)
74. 3,4-methylenedioxy-6-propylbenzyl N-butyldiethyleneglycol Ether
75. 5-propyl-4-(2,5,8-trioxa-dodecyl)-1,3-benzodioxol [german]
76. Piperonyl Butoxide (pbo)
77. 3,4-methylendioxy-6-propylbenzyl-n-butyl-diaethylenglykolaether [german]
78. Alpha-(2-(2-butoxyethoxy)ethoxy)-4,5-methylenedioxy-2-propyltoluene
79. 3,4-methylenedioxy-6-propylbenzyl-n-butyl-diaethylenglykolaether [german]
80. Ec 200-076-7
81. Toluene, Alpha-(2-(2-butoxyethoxy)ethoxy)-4,5-(methylenedioxy)-2-propyl-
82. Schembl5490
83. 4-19-00-00779 (beilstein Handbook Reference)
84. 5-propyl-4-(2,5,8-trioxa-dodecyl)-1,3-benzodioxol
85. Anvil 2+2 Ulv (salt/mix)
86. Piperonyl Butoxide [mi]
87. Chembl1201131
88. Piperonyl Butoxide [iso]
89. Nsc8401
90. Piperonyl Butoxide [hsdb]
91. Piperonyl Butoxide [iarc]
92. Piperonyl Butoxide [inci]
93. Bdbm181115
94. Hms3264a07
95. Piperonyl Butoxide [vandf]
96. Us9138393, Piperonyl Butoxide
97. Us9144538, Piperonyl Butoxide
98. Anvil 10+10 Ulv (salt/mix)
99. Piperonyl Butoxide [mart.]
100. 3,4-methylenedioxy-6-propylbenzyl-n-butyl-diaethylenglykolaether
101. Bcp19227
102. Fac 5273
103. Hy-b1198
104. Piperonyl Butoxide [who-dd]
105. Zinc3875342
106. Tox21_111034
107. Tox21_400086
108. Mfcd00005842
109. S4831
110. Akos015951348
111. Toluene,5-(methylenedioxy)-2-propyl-
112. Tox21_111034_1
113. Ccg-213921
114. Cs-4826
115. Db09350
116. Piperonyl Butoxide [green Book]
117. 2-(2-butoxyethoxy)-1-[(6-propyl(2h-benzo[d]1,3-dioxolen-5-yl))methoxy]ethane
118. Piperonyl Butoxide [orange Book]
119. Ncgc00090874-01
120. Ncgc00090874-03
121. Ncgc00090874-05
122. Ncgc00090874-06
123. Piperonylbutoxide, Technical Grade, 90%
124. Ac-11663
125. As-10480
126. Db-051886
127. Piperonyl Butoxide Technical [vandf]
128. Piperonylbutoxide, Technical, >=90% (gc)
129. Ft-0631218
130. P0458
131. Wln: T56 Bo Do Chj G3 H1o2o2o4
132. Piperonyl Butoxide 100 Microg/ml In Methanol
133. Rid Mousse Component Piperonyl Butoxide
134. C18880
135. D08383
136. Piperonyl Butoxide 10 Microg/ml In Cyclohexane
137. Piperonyl Butoxide 1000 Microg/ml In N-hexane
138. S11735
139. Ab01563216_01
140. Piperonyl Butoxide 10 Microg/ml In Acetonitrile
141. Pesticide4_piperonyl Butoxide_c19h30o5_butacide
142. Piperonyl Butoxide 100 Microg/ml In Acetonitrile
143. Piperonyl Butoxide Component Of Rid Mousse
144. Q420891
145. Sr-01000944266
146. 5-propyl-4-(2,8-trioxa-dodecyl)-1,3-benzodioxol
147. Piperonylbutoxide, Pestanal(r), Analytical Standard
148. Q-201588
149. Sr-01000944266-1
150. 1, 5-[[2-(2-butoxyethoxy)ethoxy]methyl]-6-propyl-
151. 4,5-methylenedioxy-2-propylbenzyldiethylene Glycol Butyl Ether
152. Nsc 8401; Nsc-8401; Nsc8401; Ent-14250
153. Piperonylbutoxide, Certified Reference Material, Tracecert(r)
154. (3,4-methylenedioxy-6-propylbenzyl) (butyl) Diethylene Glicol Ether
155. 5-([2-(2-butoxyethoxy)ethoxy]methyl)-6-propyl-1,3-benzodioxole #
156. Piperonylbutoxide, British Pharmacopoeia (bp) Reference Standard
157. (butylcarbityl)(6-propylpiperonyl) Ether 80% And Related Compounds 20%
158. 1,3-benzodioxole, 5-((2-(2-butoxyethoxy)ethoxy)methyl)-6-propyl
159. 5((2-(2-butoxyethoxy)ethoxy)methyl)-6-propyl-1,3-benzodioxole
160. Toluene, .alpha.-[2-(2-butoxyethoxy)ethoxy]-4,5-(methylenedioxy)-2-propyl-
161. 2-(2-butoxyethoxy)ethyl (6-propylpiperonyl) Ether, 4,5-methylenedioxy-2-propylbenzyldiethyleneglycol Butyl Ether
| Molecular Weight | 338.4 g/mol |
|---|---|
| Molecular Formula | C19H30O5 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 13 |
| Exact Mass | 338.20932405 g/mol |
| Monoisotopic Mass | 338.20932405 g/mol |
| Topological Polar Surface Area | 46.2 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 312 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Piperonyl butoxide itself has no known therapeutic use. ... Formulations of pyrethrins containing piperonyl butoxide are used as a pediculicide to control the body louse Pediculus humanus humanus, the head louse, P. humanus capitus, and the crab louse Pthirus pubis ... .
Hayes, W.J., Jr., E.R. Laws, Jr., (eds.). Handbook of Pesticide Toxicology. Volume 3. Classes of Pesticides. New York, NY: Academic Press, Inc., 1991., p. 1514
2. 2 = slightly toxic: probable oral lethal dose (human) 5-15 g/kg, between 1 pint and 1 quart for a 70 kg person (150 lb).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-310
For the treatment of head, pubic (crab), and body lice.
Piperonyl butoxide does not affect the mixed-function oxidase system in humans. In small trials in human volunteers, usual doses of piperonyl butoxide had no effect on humans.
Pesticide Synergists
Chemicals that, while not possessing inherent pesticidal activity, nonetheless promote or enhance the effectiveness of other pesticides when combined. (See all compounds classified as Pesticide Synergists.)
Absorption
Piperonyl butoxide is applied topically. In a study evaluating the 7-day urinary accumulation of piperonyl butoxide after topical application, it was found that approximately 2% of the dose was absorbed through the skin. The percutaneous absorption when applied to the scalp was found to be 8.3%.
Route of Elimination
In an absorption study in human volunteers, it was found that, if absorbed, piperonyl butoxide was eliminated in urine.
Volume of Distribution
Piperonyl butoxide is minimally absorbed in humans. Volume of distribution has not been studied.
Clearance
Clearance of piperonyl butoxide has not been studied.
No ... significant percutaneous absorption /in test mammals/.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-310
Distribution of radioactivity showed that the brain and thoracic ganglia, fore- and hind-gut, and Malpighian tubules of the kidney contained greatest amt of ... /(14)C-labeled piperonyl butoxide/ per unit wt ... /in Madeira roaches/.
Menzie, C.M. Metabolism of Pesticides. U.S. Department of the Interior, Bureau of Sport Fisheries and Wildlife, Publication 127. Washington, DC: U.S. Government Printing Office, 1969., p. 251
Piperonyl butoxide is poorly absorbed from GI tract. In 2 experiments, 78% & 87%, respectively, of dose admin orally to dogs were recovered in feces. The small proportion that was absorbed from GI tract was rapidly excreted in urine. Intratracheal admin led to more prolonged excretion of metabolites in the bile and urine, but even in this instance residues in lung tissue were less than they were following iv admin.
Hayes, Wayland J., Jr. Pesticides Studied in Man. Baltimore/London: Williams and Wilkins, 1982., p. 115
... 48 hr after oral admin of ... (14)C-piperonyl butoxide ... to mice, 76% of (14)C had been excreted in expired air, 7% in urine, and 4% in feces ... In rats, about 40% was excreted as (14)C-CO2 in expired air, 8 hr after iv dose ...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 85
For more Absorption, Distribution and Excretion (Complete) data for Piperonyl butoxide (9 total), please visit the HSDB record page.
Piperonyl butoxide is minimally absorbed in humans. Metabolism has not been studied.
[14C]-piperonyl butoxide (PBO) was administered to male and female rats by gavage at a dose rate of 50 or 500 mg/kg body weight. In all cases, the radioactivity was rapidly excreted with 87-99% being found in the 0-48-hr excreta and the majority of the dose (64.1-85.0%) being eliminated in feces. The metabolism of PBO was complex with over 25 peaks of radioactivity being seen by radio-high-performance liquid chromatography (HPLC). Using HPLC/tandem mass spectrometry (MS/MS) and nuclear magnetic resonance (NMR), 12 urine metabolites were assigned structures together with four plus PBO in feces. Metabolism occurred at two sites: the methylenedioxy ring, which opened to form a catechol that could then undergo methylation, and the 2-(2-butoxyethoxy)ethoxymethyl side-chain, which underwent sequential oxidation to a series of alcohols and acids. The identified metabolites accounted for approximately 60% of the administered dose.
Byard J, Needham D; Xenobiotica 36 (12) 1259-72 (2006). Available from, as of October 16, 2009: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=17162471
In mice, the major metabolic pathway for piperonyl butoxide ... incl cleavage of the methylenedioxyphenol residue and exhalation of methylene carbon atom as CO2. Products in urine ... comprise many compounds without methylenedioxyphenyl residue plus small amt of 6-propylpiperonylic acid and its glycine conjugate ...
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 304
In mammals (and also in insects), oxidative attack on the carbon atom of the methylenedioxy group leads to the formation of the dihydroxyphenyl compound. Oxidative degradation of the side-chain also occurs.
Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 818
In a metabolism study, a mixture of non-radiolabeled (93.4% a.i.) and phenyl labeled 14C-piperonyl butoxide (100% radiochemical purity) was administered to 4 CRL:CD rats/sex/dose by single gavage exposure at dose levels of 50 or 500 mg/kg body weight. The main route of excretion was via feces which contained 82.9-85.1% of the administered radioactivity at the low dose level and 64.1-75.9% at the high dose level at 168 hours. The percent radioactive dose excreted in the urine during 168 hours was 11.1-14.4% in low dose group and 19.5-30.2% in the high dose group. The majority of the administered radioactivity was excreted in 0-48 hour urine and feces samples in both dose groups. The percent of administered dose in carcass was below 0.5% in either low dose or high dose groups. The total percent of radioactive dose recovered in both dose groups ranged between 97.4% and 99.6%. There is no significant difference in the excretion pattern either between two dose groups or between sexes in the same dose group. M1 and M3 are the major metabolites excreted in feces. M1 was identified as unchanged PBO corresponding to 15.6-23.9% of administered dose. M3 was identified as PBO with methylenedioxy ring opened to form catechol and found at 17.4-19.7% of the administered dose. M2 and M4/M5 were also identified but were present in low amounts (4-6% of administered radioactivity) in high dose group. Several radioactive peaks (~20 peaks) were observed in urine samples and none of these individual peaks exceeded 5% of the administered radioactivity. The significant metabolite in male urine was found to be M14 which occurred at 3% of the administered dose. In females, the significant urinary metabolites identified were M6 and the combined M7/M8 which contained 5 and 9% of the administered dose, respectively. Although there was no significant difference in the excretion of metabolites between two dose groups, metabolites M5, M8, M9 and M10 were predominantly found in female urine samples and M14 was reported only in male urine samples. Based on the identification of metabolites, the authors proposed three major reactions in the metabolism of PBO: 1) Opening of the methylenedioxy ring to form the catechol; 2) Sequential cleavage of the 2-(2-butoxyethoxy)ethoxymethyl side chain to produce series of alcohols and acids; 3) Conjugation of one of the phenolic groups to yield glucuronide, sulfate or methoxy derivative.
USEPA/Office of Pesticide Programs; Piperonyl Butoxide: Revised Metabolism Assessement Review Committee Report p.44 Identification Number: EPA-HQ-OPP-2005-0042 (September 2005). Available from, as of October 15, 2009: https://www.regulations.gov/search/Regs/home.html#home
32 hours.
In order to determine the human in vivo percutaneous absorption of piperonyl butoxide, a commercial formulation containing (14C)piperonyl butoxide (3.4 mCi/uM) was applied to the ventral forearm of six human volunteers. The formulation contained 3.0% piperonyl butoxide. Spreadability studies showed that concn 75.8 ug piperonyl butoxide/sq cm (used in this study) would be consistent with levels found in actual use. The forearms were thoroughly cleansed with soap and water 30 min after application (as recommended for actual use). Percutaneous absorption was determined by urinary cumulative excretion following dose application. With a 7 day urinary accumulation, ... 2.1+/- 0.6% of the dose of piperonyl butoxide applied was absorbed through the forearm skin. 1 hr after application blood samples contained no detectable radioactivity. The percutaneous absorption ... of piperonyl butoxide from the scalp was calculated to be 8.3%. ... The calculated half life of (14)C excretion was 32 hr for piperonyl butoxide. ...
PMID:8132164 Wester RC et al; Food Chem Toxicol 32 (1): 51-3 (1994)
Piperonyl butoxide is not a pesticide, but acts as a synergist to increase the activity of pesticides such as carbamates, pyrethrins, pyrethroids, and rotenone. Piperonyl butoxide inhibits the mixed-function oxidase (MFO) system of an insect. The MFO system is the insects natural defense system and causes the oxidative breakdown of insecticides. Thus, by inhibiting this system piperonyl butoxide promotes higher levels of insecticide and allows for lower doses to be used for a lethal effect.
To clarify the mechanism of piperonyl butoxide (PBO)-induced hepatocarcinogenesis in mice, male mice were subjected to a two-thirds partial hepatectomy, N-diethylnitrosamine (DEN) initiation, and a diet containing 0.6% PBO for eight weeks. The incidence of gamma-glutamyl transpeptidase (GGT)-positive foci and PCNA-positive cells was significantly increased in the DEN + PBO group compared with the DEN-alone group. Real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis showed up-regulation of genes related to metabolism, such as cytochrome P450 1A1 and 2B10, and metabolic stress, such as Por, Nqo1, Nrf2, abcc3, and abcc4. Early responsive genes downstream of mitogen-activated protein kinase (MAPK), such as c-fos, c-jun, c-myc, and activating transcription factor 3 (ATF3), were also up-regulated in this group. Positive immunohistochemical staining for ATF3 was diffusely observed in nonproliferating hepatocytes of the DEN + PBO group, but altered foci were negative or weakly positive for ATF3. The nuclei of hepatocytes within ATF3-negative foci were positive for cyclin D. Thus PBO can induce oxidative stress, activate the MAPK pathway, and increase ATF3 transcript levels in hepatocytes outside the altered foci during the early stage of PBO-induced hepatocarcinogenesis in mice. .
Kawai M et al; Toxicol Pathol 37 (6): 761-9 (2009). Available from, as of October 16, 2009: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=19690152
... Male F344 rats were administered piperonyl butoxide mixed in the diet at concentrations of 0 (negative control), 0.05, 0.2 or 2% for 2 days, 1, 2, and 4 weeks. As a positive control, phenobarbital was administered to rats for up to 4 weeks as a 0.1% solution in the drinking water. Increased liver weight, centrilobular hepatocellular hypertrophy due to increased smooth endoplasmic reticulum, decreased numbers and areas of connexin 32-positive spots per hepatocyte, and increased cell proliferation were observed in rats treated with 0.2 and 2% piperonyl butoxide. Similar results were obtained for 0.1% phenobarbital treated rats. Hepatocellular necrosis suggestive of hepatotoxicity was also observed in the 2% piperonyl butoxide group. These results indicate that the promoting mechanism of piperonyl butoxide in hepatocarcinogenesis is similar to that of phenobarbital, involving an ability to induce CYP isoenzymes and inhibit gap junctional intercellular communication. In addition, increased cell proliferation following hepatocellular necrosis may also play a role at high doses.
Okamiya H et al; Arch Toxicol 72 (11): 744-50 (1998). Available from, as of October 16, 2009: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=9879813
Piperonyl butoxide (PBO) is an insecticide synergist known to inhibit the activity of cytochrome P450 enzymes. ... Little is known about how insects respond to PBO exposure at the gene transcription level. The authors have characterized the transcriptional response of the Drosophila melanogaster genome after PBO treatment, using both a custom-designed 'detox' microarray, containing cytochrome P450 (P450), glutathione S-transferase (GST) and esterase genes, and a full genome microarray. A subset of P450 and GST genes is identified, along with additional metabolic genes, that are induced by PBO. The gene set is an extremely similar gene set to that induced by phenobarbital, a compound for which pretreatment is known to confer tolerance to a range of insecticide compounds.
Willoughby L et al; Pest Management Sci 63 (8): 803-8 (2007). Available from, as of October 16, 2009: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=17514638
Piperonyl butoxide (PBO), alpha-[2-(2-butoxyethoxy)ethoxy]-4,5-methylene-dioxy-2-propyltoluene, is widely used as a synergist for pyrethrins. In order to clarify the possible mechanism of non-genotoxic hepatocarcinogenesis induced by PBO, molecular pathological analyses consisting of low-density microarray analysis and real-time reverse transcriptase (RT)-PCR were performed in male ICR mice fed a basal powdered diet containing 6000 or 0 ppm PBO for 1, 4, or 8 weeks. The animals were sacrificed at weeks 1, 4, and 8, and the livers were histopathologically examined and analyzed for gene expression using the microarray at weeks 1 and 4 followed by real-time RT-PCR at each time point. Reactive oxygen species (ROS) products were also measured using liver microsomes. At each time point, the hepatocytes of PBO-treated mice showed centrilobular hypertrophy and increased lipofuscin deposition in Schmorl staining. The ROS products were significantly increased in the liver microsomes of PBO-treated mice. In the microarray analysis, the expression of oxidative and metabolic stress-related genes--cytochrome P450 (Cyp) 1A1, Cyp2A5 (week 1 only), Cyp2B9, Cyp2B10, and NADPH-cytochrome P450 oxidoreductase (Por) was over-expressed in mice given PBO at weeks 1 and 4. Fluctuations of these genes were confirmed by real-time RT-PCR in PBO-treated mice at each time point. In additional real-time RT-PCR, the expression of Cyclin D1 gene, key regulator of cell-cycle progression, and Xrcc5 gene, DNA damage repair-related gene, was significantly increased at each time point and at week 8, respectively. These results suggest the possibility that PBO has the potential to generate ROS via the metabolic pathway and to induce oxidative stress, including oxidative DNA damage, resulting in the induction of hepatocellular tumors in mice.
Muguruma M et al; Toxicology 228 (2-3): 178-87 (2006). Available from, as of October 16, 2009: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=17014948




