
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
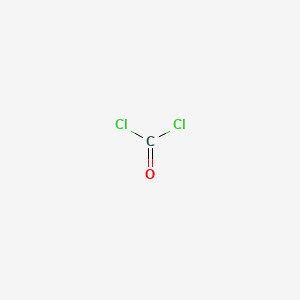

1. Carbonyl Dichloride
2. Carbonic Dichloride
3. Carbonyl Chloride
4. Phosgen
5. Chloroformyl Chloride
6. Carbon Oxychloride
7. 75-44-5
8. Carbonic Chloride
9. Carbonylchlorid
10. Carbon Dichloride Oxide
11. Fosgeen
12. Fosgen
13. Carbonic Acid Dichloride
14. Dichloroformaldehyde
15. Carbone (oxychlorure De)
16. Fosgene
17. Koolstofoxychloride
18. Rcra Waste Number P095
19. Chloroketone
20. Carbonio (ossicloruro Di)
21. Nci-c60219
22. Un 1076
23. Phosgene Solution, 20% In Toluene
24. Combat Gas
25. Fosgeen [dutch]
26. Fosgen [polish]
27. Phosgen [german]
28. Fosgene [italian]
29. 117k140075
30. Carbonylchlorid [german]
31. Koolstofoxychloride [dutch]
32. Hsdb 796
33. Carbone (oxychlorure De) [french]
34. Einecs 200-870-3
35. Carbonio (ossicloruro Di) [italian]
36. Un1076
37. Rcra Waste No. P095
38. Brn 1098367
39. Dichloroketone
40. Carbonylchloride
41. Chloro Ketone
42. Unii-117k140075
43. Phosgene [hsdb]
44. Phosgene [mi]
45. Phosgene [mart.]
46. C O Cl2
47. Ec 200-870-3
48. Phosgene, 20% In Toluene
49. 4-03-00-00031 (beilstein Handbook Reference)
50. Schembl4685827
51. Dtxsid0024260
52. Chebi:29365
53. Mfcd00036119
54. Phosgene Solution, ~20% In Toluene
55. Zinc59372684
56. Phosgene [un1076] [poison Gas]
57. Akos015915019
58. Phosgene Solution, 15 Wt. % In Toluene
59. Q189090
| Molecular Weight | 98.91 g/mol |
|---|---|
| Molecular Formula | CCl2O |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 97.9326200 g/mol |
| Monoisotopic Mass | 97.9326200 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 29 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
LC50 humans approx 500 ppm/ 1 min ... exposure at 3 ppm for 170 min was equally as fatal as exposure at 30 ppm for 17 min.
American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-1634 2006.
The LC50 in humans is estimated at 500 to 800 ppm for one minute.
IPCS; Poisons Information Monograph 419. Phosgene. (October 1997). Available from, as of July 16, 2007: https://www.inchem.org/documents/pims/chemical/pim419.htm
Concn greater than 0.25 mg/L of air (62 ppm) may be fatal, when breathed for one-half hour or more. Three to 5 mg/L causes death within a few minutes.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 192
Chemical Warfare Agents
Chemicals that are used to cause the disturbance, disease, or death of humans during WARFARE. (See all compounds classified as Chemical Warfare Agents.)
Rats were exposed to 0.1 - 1.0 ppm 14C-phosgene (0.4 - 4.1 mg/cu m) for 0.5 to 4 hr and tissue distribution of carbon-14 was investigated. Phosgene adduct concentrations (measured as nmol carbon-14/g dry weight) were highest in the lung, trachea, nasopharynx and blood, and were also highly concentrated in constituents of nasal and broncheolar lavage fluids. Lung tissue carbon-14 was distributed in lipid, macromolecule, and water-soluble fractions in a manner which was proportional to the weight contributed by each fraction.
European Chemicals Bureau; IUCLID Dataset, Phosgene (75-55-5) p. 29 (2000 CD-ROM edition). Available from, as of July 16, 2007: https://esis.jrc.ec.europa.eu/
Rabbits, guinea pigs, rats, hamsters and mice were exposed simultaneously to 1.6 ppm 14C-phosgene (6.6 mg/cu m) for 3 min in a closed Plexiglas chamber. Mean lung concentrations of carbon-14 in these animals immediately following exposure were 3.15, 2.50, 5.33, 5.05 and 11.13 nmoles/g wet weight, respectively. Blood carbon-14 ranged from 2.5% of lung concentrations in rabbits to 5.9% in mice. The nasopharyngeal fraction of total respiratory tract carbon-14 was higher in rabbits and guinea pigs (0.46 - 0.50) than in the other species (0.22 - 0.36). Tracheal carbon-14 was always about 3% of total respiratory tract carbon-14. Liver carbon-14 was about 1% of lung concentrations. Tissue binding of phosgene was shown by a large percentage of carbon-14 in trichloroacetic acid precipitates of tissue and by the presence of carbon-14 in lungs (20% of initial) 3 days post exposure.
European Chemicals Bureau; IUCLID Dataset, Phosgene (75-55-5) p. 30 (2000 CD-ROM edition). Available from, as of July 16, 2007: https://esis.jrc.ec.europa.eu/
The gas penetrates into the tissues of respiratory tract and to some extent is absorbed by the lung. ... Hydrochloric acid and carbon dioxide produced by hydrolysis of phosgene are excreted largely by kidney and lung, respectively.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 193
Rats, mice, hamsters, guinea pigs and rabbits were exposed by inhalation to 14C-phosgene at 1.6 ppm for 3 minutes. Carbon-14 was detected at very low levels in blood and liver samples of all animals.
USEPA; Health Assessment Document: Phosgene p.3-3 (1986) EPA-600/8-86-022A
Cysteine inhibited in vitro covalent binding of chloroform to liver microsomal protein and trapped a reactive metabolite, presumably phosgene, as 2-oxothiazolidine-4-carboxylic acid. This suggests that the carbon-hydrogen bond of chloroform is oxidized by a cytochrome P450 monooxygenase to produce trichloromethanol, which spontaneously dehydrochlorinates to yield phosgene.
PMID:597296 Pohl LR et al; Biochem Biophys Res Commun 79 (3): 684-91 (1977)
Phosgene is formed during NADPH and oxygen dependent microsomal oxidation of /carbon tetrachloride/.
PMID:588283 Mansay D et al; Biochem Biophys Res Comm 79 (2): 513-7 (1977)
Phosgene reacts rapidly with water, but more rapidly with certain chemical groups found in tissue macromolecules, such as free amines and sulfhydryls. Because of its high reactivity, it is doubtful that any unreacted phosgene will enter the general circulation even after exposure to high concentrations of the gas.
USEPA; Health Assessment Document: Phosgene p.3-9 (1986) EPA-600/8-86-022A
... Sensitivity to chloroform correlates with the capacity of the kidney to metabolize chloroform to the toxic metabolite phosgene. ... Kidney homogenates of sensitive male DBA/2J mice metabolized chloroform to phosgene more rapidly than did the less sensitive C57BL/6J mice. Similarly, kidney homogenates from male mice, which are sensitive to chloroform induced nephrotoxicity, metabolized chloroform to phosgene at nearly an order of magnitude more rapidly than did those from female mice. Treatment of female mice with testosterone, however, reversed this trend. Cytochrome P450 in the microsomal and mitochondrial fraction of the kidney appeared to catalyze the metabolism of chloroform to phosgene.
PMID:6145557 Pohl LR et al; Drug Metab Dispos 12 (3): 304-8 (1984)
No reports found; [TDR, p. 1030]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 1030
Phosgene is a highly reactive gas capable of damaging a variety of biological macromolecules in an oxidant-like fashion. This activity potentially results from at least two separate chemical reactions: acylation and hydrolysis. Acylation, the more important and rapid mechanism, results from the reaction of phosgene with nucleophilic moieties, such as the amino, hydroxyl, and sulfhydryl groups of tissue macromolecules. Acylation causes destruction of proteins and lipids, irreversible alterations of membrane structures, and disruption of enzyme and other cell functions. Exposure to phosgene depletes lung nucleophiles, particularly glutathione, and restoration of glutathione seems to protect against phosgene-induced injury. For several days after acute phosgene exposure, tissue levels of antioxidant enzymes, such as glutathione reductase and superoxide dismutase, increase as part of the lungs' response to injury. In addition to acylation, phosgene is hydrolyzed to HCl ... The formation of HCl occurs on moist membranes and may cause irritation and tissue damage. Because of the limited water solubility of phosgene, it is unlikely that large quantities of HCl could result from the exposure of biological tissues. However, small amounts do form and may contact moist membranes of the eye, nasopharynx, and respiratory tract. Hydrolysis to HCl is the probable cause of immediate inflammation and discomfort after phosgene exposure at concentrations greater than 3 ppm (>12 mg/cu m). Pulmonary cellular glycolysis and oxygen uptake following phosgene exposure are depressed and, thus, leads to a corresponding decrease in the levels of intracellular adenosine triphosphate and cyclic adenosine monophosphate. This is associated with increased water uptake by epithelial, interstitial, and endothelial cells. The semipermeability of the blood-air barrier becomes gradually compromised as a result of fluid entering the interstitial and alveolar spaces. Later, the blood-air barrier disrupts, opening channels for the flooding of alveoli. Compression of pulmonary microvasculature leads to the opening of arteriovenous shunt. The onset of pulmonary edema correlates temporally with the decrease in adenosine triphosphate levels. Interventions that increase intracellular cyclic adenosine monophosphate, such as treatment with phosphodiesterase inhibitors (e.g., aminophylline), beta-adrenergic agonists (e.g., isoproterenol), or cyclic adenosine monophosphate analogs, markedly reduce pulmonary edema formation in animals exposed to phosgene.
USEPA; TOXICOLOGICAL REVIEW OF PHOSGENE (CAS No. 75-44-5) In Support of Summary Information on the Integrated Risk Information System (IRIS) p. 19-20. EPA/635/R-06/001 www.epa.gov/iris (December 2005)
The pathology of phosgene poisoning is mainly due to its acylating properties and not a result of hydrochloric acid generation upon hydrolysis, although hydrochloric acid production may play a minor role. The production of pulmonary edema following phosgene exposure has been correlated with reductions in pulmonary ATP levels and sodium-potassium ATPase activity, as well as inhibition of other pulmonary enzymes. The role of the nervous system in the toxicity of phosgene is considered to be a nonspecific effect of irritant gases.
USEPA; Health Assessment Document: Phosgene p.3-9 (1986) EPA-600/8-86-022A
Phosgene is only slightly soluble in water; the small amount of phosgene which dissolves is immediately hydrolyzed to CO2 and HCl. The concentration of HCl produced from a lethal dose of phosgene is
European Chemicals Bureau; IUCLID Dataset, Phosgene (75-55-5) p. 30 (2000 CD-ROM edition). Available from, as of July 16, 2007: https://esis.jrc.ec.europa.eu/

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
ABOUT THIS PAGE
65
PharmaCompass offers a list of Phosgene API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Phosgene manufacturer or Phosgene supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Phosgene manufacturer or Phosgene supplier.
PharmaCompass also assists you with knowing the Phosgene API Price utilized in the formulation of products. Phosgene API Price is not always fixed or binding as the Phosgene Price is obtained through a variety of data sources. The Phosgene Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Phosgene manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Phosgene, including repackagers and relabelers. The FDA regulates Phosgene manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Phosgene API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Phosgene supplier is an individual or a company that provides Phosgene active pharmaceutical ingredient (API) or Phosgene finished formulations upon request. The Phosgene suppliers may include Phosgene API manufacturers, exporters, distributors and traders.
click here to find a list of Phosgene suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Phosgene DMF (Drug Master File) is a document detailing the whole manufacturing process of Phosgene active pharmaceutical ingredient (API) in detail. Different forms of Phosgene DMFs exist exist since differing nations have different regulations, such as Phosgene USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Phosgene DMF submitted to regulatory agencies in the US is known as a USDMF. Phosgene USDMF includes data on Phosgene's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Phosgene USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Phosgene suppliers with USDMF on PharmaCompass.
Phosgene Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Phosgene GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Phosgene GMP manufacturer or Phosgene GMP API supplier for your needs.
A Phosgene CoA (Certificate of Analysis) is a formal document that attests to Phosgene's compliance with Phosgene specifications and serves as a tool for batch-level quality control.
Phosgene CoA mostly includes findings from lab analyses of a specific batch. For each Phosgene CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Phosgene may be tested according to a variety of international standards, such as European Pharmacopoeia (Phosgene EP), Phosgene JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Phosgene USP).

