


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
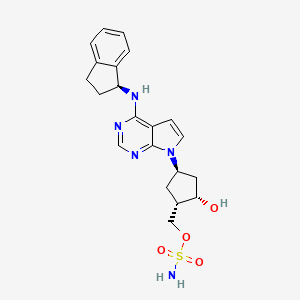

1. ((1s,2s,4r)-4-(4-((1s)-2,3-dihydro-1h-inden-1-ylamino)-7h-pyrrolo(2,3-d)pyrimidin-7-yl)-2-hydroxycyclopentyl)methyl Sulfamate
2. ((1s,2s,4r)-4-(4-((1s)-2,3-dihydro-1h-inden-1-ylamino)-7h-pyrrolo(2,3-d)pyrimidin-7-yl)-2-hydroxycyclopentyl)methyl Sulphamate
3. 4-(4-((1s)-2,3-dihydro-1h-inden-1-ylamino)-7h-pyrrolo(2,3-d)pyrimidin-7-yl)-2-hydroxycyclopentyl Methyl Sulfamidate
4. Mln-4924
5. Mln4924
1. Mln4924
2. Mln-4924
3. 905579-51-3
4. Mln 4924
5. Pevonedistat (mln-4924)
6. ((1s,2s,4r)-4-(4-(((s)-2,3-dihydro-1h-inden-1-yl)amino)-7h-pyrrolo[2,3-d]pyrimidin-7-yl)-2-hydroxycyclopentyl)methyl Sulfamate
7. Mln-4924003
8. S3azd8d215
9. 905579-51-3 (free Base)
10. Pevonedistat (usan)
11. Pevonedistat [usan]
12. [(1s,2s,4r)-4-{4-[(1s)-2,3-dihydro-1h-inden-1-ylamino]-7h-pyrrolo[2,3-d]pyrimidin-7-yl}-2-hydroxycyclopentyl]methyl Sulfamate
13. Sulfamic Acid [(1s,2s,4r)-4-[4-[[(1s)-2,3-dihydro-1h-inden-1-yl]amino]-7h-pyrrolo[2,3-d]pyrimidin-7-yl]-2-hydroxycyclopentyl]methyl Ester
14. ((1s,2s,4r)-4-(4-((1s)-2,3-dihydro-1h-inden-1-ylamino)-7h-pyrrolo(2,3-d)pyrimidin-7-yl)-2-hydroxycyclopentyl)methyl Sulfamate
15. Sulfamic Acid, ((1s,2s,4r)-4-(4-(((1s)-2,3-dihydro-1h-inden-1-yl)amino)-7h-pyrrolo(2,3-d)pyrimidin-7-yl)-2-hydroxycyclopentyl)methyl Ester
16. Pevonedistat [usan:inn]
17. Pevonedistatum
18. Unii-s3azd8d215
19. 3gzn
20. ((1s,2s,4r)-4-{4-[(1s)-2,3-dihydro-1h-inden-1-ylamino]-7h-pyrrolo[2,3-d]pyrimidin-7-yl}-2-hydroxycyclopentyl)methyl Sulfamate
21. B39
22. Pevonedistat; Mln4924
23. Pevonedistat [inn]
24. ((1s,2s,4r)-4-(4-((1s)-2,3-dihydro-1h-inden-1-ylamino)-7h-pyrrolo(2,3-d)pyrimidin-7-yl)-2-hydroxycyclopentyl)methyl Sulphamate
25. Mls006010830
26. Pevonedistat [who-dd]
27. Schembl192875
28. Tak924
29. Chembl1231160
30. Chebi:95028
31. Gtpl12117
32. Tak-924
33. Chebi:145535
34. Dtxsid701022549
35. Ex-a1472
36. Bdbm50285607
37. Nsc793435
38. Zinc58660702
39. Akos025401927
40. Cs-0348
41. Db11759
42. Nsc-793435
43. Ncgc00345794-02
44. Ncgc00345794-07
45. Ncgc00345794-10
46. [(1s,2s,4r)-4-[4-[[(1s)-2,3-dihydro-1h-inden-1-yl]amino]pyrrolo[2,3-d]pyrimidin-7-yl]-2-hydroxycyclopentyl]methyl Sulfamate
47. [(1s,2s,4r)-4-{4-[(1s)-2,3-dihydro-1h-inden-1-ylamino]-7h-pyrrolo [2,3-d]pyrimidin-7-yl}-2-hydroxycyclopentyl]methyl Sulfamate
48. 4-(4-((1s)-2,3-dihydro-1h-inden-1-ylamino)-7h-pyrrolo(2,3-d)pyrimidin-7-yl)-2-hydroxycyclopentyl Methyl Sulfamidate
49. Ac-27394
50. Hy-70062
51. Smr004701757
52. D10413
53. Brd-k67844266-001-01-3
54. Q27166792
55. ((1s,2s,4r)-4-(4-(((1s)-2,3-dihydro-1h-inden-1-yl)amino(-7h-pyrrolo(2,3-d)pyrimidin-7-yl)-2-hydroxycyclopentyl)methyl Sulfamate
56. ((1s,2s,4r)-4-(4-((s)-2,3-dihydro-1h-inden-1-ylamino)-7h-pyrrolo[2,3-d]pyrimidin-7-yl)-2-hydroxycyclopentyl)methyl Sulfamate
57. ((1s,2s,4r)-4-{4-[(1s)-2,3-dihydro-1h-inden-1-ylamino]-7h-pyrrolo[2,3-d]-pyrimidin-7-yl}-2-hydroxycyclopentyl)methyl Sulfamate
58. [(1s,2s,4r)-4-[4-[[(1s)-2,3-dihydro-1h-inden-1-yl]amino]pyrrolo[2,3-d]pyrimidin-7-yl]-2-hydroxy-cyclopentyl]methyl Sulfamate
| Molecular Weight | 443.5 g/mol |
|---|---|
| Molecular Formula | C21H25N5O4S |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 443.16272547 g/mol |
| Monoisotopic Mass | 443.16272547 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 734 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of acute myeloid leukaemia, Treatment of myelodysplastic syndromes
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)


