



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Ai 700
2. Ai-700
3. Ai700 Compound
4. Decafluorobutane
5. Mp 1950
6. Mp-1950
7. Mp1950
8. Perfluorobutane
1. Decafluorobutane
2. Perfluorobutane
3. 355-25-9
4. Sonazoid
5. Perfluoro-n-butane
6. Butane, Decafluoro-
7. 1,1,1,2,2,3,3,4,4,4-decafluorobutane
8. Ai-700
9. Se4twr0k2c
10. Decafluoro-butane
11. Cea 410
12. Einecs 206-580-3
13. Unii-se4twr0k2c
14. Ai 700
15. Perflubutane [usan:inn]
16. Fc 3110
17. Pf 5040
18. Perflubutano
19. Perflubutanum
20. Nc 100100
21. Hsdb 7868
22. Sonazoid (tn)
23. Pfc 31-10
24. Perflubutane [inn]
25. Perflubutane [jan]
26. Perflubutane [usan]
27. Perflubutane [mart.]
28. Perflubutane [who-dd]
29. Schembl111514
30. Perflubutane (jan/usan/inn)
31. Chembl2104979
32. Dtxsid5059876
33. Chebi:134964
34. Mfcd00042080
35. Zinc56897668
36. Akos015852695
37. Db12821
38. Db-048827
39. Ft-0624477
40. D05440
41. 1,1,1,2,2,3,3,4,4,4-decafluorobutane #
42. Butane, 1,1,1,2,2,3,3,4,4,4-decafluoro-
43. Q3375325
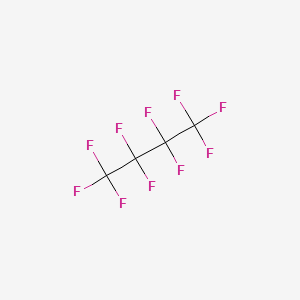
| Molecular Weight | 238.03 g/mol |
|---|---|
| Molecular Formula | C4F10 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 1 |
| Exact Mass | 237.98403162 g/mol |
| Monoisotopic Mass | 237.98403162 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 181 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Perfluorobutane is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=Perfluorobutane&Search=Search
/Experimental Therapy/ Ultrasound contrast agents are known to enhance high intensity focused ultrasound (HIFU) ablation, but these perfluorocarbon microbubbles are limited to the vasculature, have a short half-life in vivo, and may result in unintended heating away from the target site. Herein, a nano-sized (100-300 nm), dual perfluorocarbon (decafluorobutane/dodecafluoropentane) droplet that is stable, is sufficiently small to extravasate, and is convertible to micron-sized bubbles upon acoustic activation was investigated. Microbubbles and nanodroplets were incorporated into tissue-mimicking acrylamide-albumin phantoms. Microbubbles or nanodroplets at 0.1 x 10(6) per cu cm resulted in mean lesion volumes of 80.4 +/- 33.1 cu mm and 52.8 +/- 14.2 cu mm (mean +/- s.e.), respectively, after 20 s of continuous 1 MHz HIFU at a peak negative pressure of 4MPa, compared to a lesion volume of 1.0 +/- 0.8 cu mm in agent-free control phantoms. Magnetic resonance thermometry mapping during HIFU confirmed undesired surface heating in phantoms containing microbubbles, whereas heating occurred at the acoustic focus of phantoms containing the nanodroplets. Maximal change in temperature at the target site was enhanced by 16.9% and 37.0% by microbubbles and nanodroplets, respectively. This perfluorocarbon nanodroplet has the potential to reduce the time to ablate tumors by one-third during focused ultrasound surgery while also safely enhancing thermal deposition at the target site.
PMID:23927187 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3745500 Phillips LC et al; J Acoust Soc Am 134 (2): 1473-82 (2013)
To explore the possibility of targeted biopsy (TBx) using transrectal ultrasound (US) with perflubutane microbubbles, we studied the findings of different cancerous tissue imaging modalities and evaluated needle biopsy in prostate cancer (PCa) using contrast-enhanced US (CEUS) in a multicenter clinical trial. Seventy-one patients undergoing prostate biopsy received intravenous injection of perflubutane microbubbles (Sonazoid). We evaluated and compared images obtained by CEUS. The safety observation period was 2 days after contrast administration. Among the 30 patients with cancer, one or more sites with findings suggestive of cancer in CEUS were detected in 23 patients (32.4%) by TBx. Although 22 patients had positive cores of cancer by systematic biopsy (SBx), 8 patients had positive cores of cancer in TBx alone (11.3%). There was a significant difference in cancer detection rate by TBx between two cohorts with PSA < 10 ng/mL (22.9%) and PSA /greater than or equal to/ 10 ng/mL (52.2%) (P < 0.02). Close observation of various CEUS findings with Sonazoid enabled targeting of cancerous areas, and consequently, a significant difference (P < 0.05) in the detection rate of cancer was recognized in the transition zone (TZ): SBx; 21/120 (17.5%) and TBx; 17/55 (30.9%). The incidence of adverse events was 6.7% and that of adverse reactions was 4%. CEUS with Sonazoid improved the detection rate of PCa by visualizing cancerous lesions. More detailed examination of CEUS images provided efficient characterization especially in the TZ area. TBx according to this procedure is expected to enable a lower number of biopsies and more accurate diagnosis of PCa.
PMID:22311543 Uemura H et al; World J Urol 31 (5): 1123-8 (2013)
Sonazoid is an ultrasound contrast agent (UCA) consisting of stabilized /perfluorobutane/ gas microbubbles in an aqueous suspension. Sonazoid has overcome the stability problems of first generation UCA and can produce myocardial perfusion images. Myocardial imaging using ultrasound contrast agents provides diagnosis of chronic heart disease and assessment of the coronary arteries and of the coronary blood flow reserve.
US-Technology Information Portal; Sonazoid. Available from, as of March 3, 2016: https://www.us-tip.com/serv1.php?type=db1&dbs=Sonazoid
Sonazoid is taken up by healthy Kupffer cells in the liver and spleen, but break down in high amplitude ultrasound imaging modes such as color Doppler imaging. The bubble rupture produces a transient pressure wave, which results in a characteristic mosaic color pattern from tissues containing the microbubbles (induced acoustic emission). Liver tumors without Kupffer cells will not display the mosaic pattern and can therefore be identified easily.
US-Technology Information Portal; Sonazoid. Available from, as of March 3, 2016: https://www.us-tip.com/serv1.php?type=db1&dbs=Sonazoid
Visualisation of myocardial perfusion for diagnostic purposes
Visualisation of myocardial perfusion for diagnostic purposes
This study has been performed to examine which cells are responsible for the hepatic clearance of the new ultrasound contrast agent Sonazoid and to study whether uptake of these gas microbubbles disturbs the function of the cells involved. Sonazoid was injected into rats and perfused fixed livers were studied by electron microscopy, which revealed that the Sonazoid microbubbles were exclusively internalized in Kupffer cells, i.e. by the macrophages located in the liver sinusoids, and not by parenchymal, stellate or endothelial cells. This is the first demonstration of intact phagocytosed gas microbubbles within Kupffer cells. Uptake of the Sonazoid perfluorobutane microbubbles by the Kupffer cells following injection of a dose corresponding to 20x the anticipated clinical dose for liver imaging did not result in measurable changes in the uptake and degradation of radioactively labelled albumin microspheres previously shown to be a useful indicator marker for Kupffer cell phagocytosis.
PMID:12712317 Kindberg GM et al; Cell Tissue Res 312 (1): 49-54 (2003)
The new ultrasound contrast agent Sonazoid was injected IV in rats at doses of 0.8 and 8 muL perfluorobutane (PFB)-containing microbubbles/kg body weight. Samples were obtained from blood, liver, spleen, fat, kidney, muscle, heart, lung and brain from both males and females and the PFB gas was analyzed using validated gas chromatography mass spectrometry methods. No differences were observed between genders or doses for any of the pharmacokinetic parameters. For all tissues, the highest concentrations were observed at the first time point (i.e., 5 min postinjection) (51% of injected dose in liver; total recovery of 69%). The highest concentrations of PFB in tissue were observed in spleen > liver > lung > kidney >> other tissues. At 24 hr after dosing, the total amount of PFB remaining in the tissues was 1.9%. These data fit well with the finding that after a Sonazoid dose of 8 microL microbubbles/kg to male rats, more than 50% of the injected PFB was recovered in exhaled air by 20 min after dosing. During the first 24 hr after administration, more than 96% of the PFB dose was recovered in exhaled air.
PMID:16364802 Toft KG et al; Ultrasound Med Biol 32 (1): 107-14 (2006)
The purpose of these studies was to determine the pharmacokinetics, tissue distribution, and exhaled elimination kinetics in rats for intravenously administered AI-700, which consists of porous microspheres containing decafluorobutane (DFB), for use as an ultrasound contrast agent. [Pd]-AI-700 was administered intravenously to rats (10 mg microspheres/kg). Blood and tissue samples collected at specified times were analyzed for palladium by inductively coupled plasma-mass spectrometry (ICP-MS). AI-700 was also administered intravenously to rats (40 mg microspheres/kg) and expired air was collected over time. Expired air samples were analyzed for DFB by validated adsorbent trapping-thermal desorption-gas chromatography-mass spectrometry methodology. Pd from [Pd]-AI-700 was cleared from blood with a ca. 50-85% decline from peak concentration within 5 min. At 1440 min post-dose, 52-72% of the Pd dose was recovered from organs of the reticuloendothelial system. Approximately 77% of the intravenously injected DFB was found in expired air within 3h after dosing, with most of the DFB dose (61+/-6%) expired within the first 10 min after dosing. As expected, the microspheres were cleared through the reticuloendothelial system, and the DFB was eliminated in expired air, with more than half of the DFB eliminated within the first 10 min after dosing.
PMID:16950578 Straub JA et al; Int J Pharm 328 (1): 35-41 (2007)
The ultrasound contrast agent Sonazoid trademark was administered as an i.v. bolus injection of 0.6 uL microbubbles/kg body weight or as a continuous infusion over 30 min at a rate of 1.2 uL microbubbles/kg body weight to healthy volunteers and patients with reduced pulmonary diffusing capacity. Expired air and blood samples were collected from 32 subjects and perfluorobutane (PFB) gas was analyzed using validated gas chromatography mass spectrometry methods. Blood concentrations of PFB declined biphasicly with a distribution half-life (t(0.5 to 15)) of 2 to 3 min and an elimination half-life (t(15 to 120)) of 30 to 45 min. Area under the curve (AUC) values in patients with impaired gas diffusion were significantly larger than those in healthy volunteers. The exhalation kinetics were somewhat variable with a PFB elimination half-life (t(15 to 120)) of 28 to 111 min. Clearance of PFB was independent of study population and mode of administration. There were no deaths and no serious adverse events that resulted in the withdrawal of a subject from the study. With the exception that arthralgia predominated in healthy volunteers, healthy volunteers and diseased subjects did not show a different adverse event profile whether Sonazoid was administered as a bolus injection or as an infusion. Assessment of laboratory parameters (serum biochemistry, hematology and urinalysis), vital signs, oxygen saturation and electrocardiograms (ECGs) showed no changes which caused safety concern.
PMID:18096304 Landmark KE et al; Ultrasound Med Biol 34 (3): 494-501 (2008)
The ultrasound contrast agent Sonazoid trademark was administered as an i.v. bolus injection of 0.6 uL microbubbles/kg body weight or as a continuous infusion over 30 min at a rate of 1.2 uL microbubbles/kg body weight to healthy volunteers and patients with reduced pulmonary diffusing capacity. ... Blood concentrations of PFB declined biphasicly with a distribution half-life (t(0.5 to 15)) of 2 to 3 min and an elimination half-life (t(15 to 120)) of 30 to 45 min. ... The exhalation kinetics were somewhat variable with a PFB elimination half-life (t(15 to 120)) of 28 to 111 min.
PMID:18096304 Landmark KE et al; Ultrasound Med Biol 34 (3): 494-501 (2008)
Perflubutane perfusion echocardiography has the potential to be a cost-effective and convenient alternative to nuclear perfusion imaging. Perflubutane is easy to use and echocardiographers in the clinical trials can be trained to use it after imaging only a few patients. Based on data from previous clinical trials, ultrasound enhanced with Perflubutane was able to image myocardial perfusion and obtain information that appears comparable to nuclear imaging. Acusphere's Phase 3 program is designed with the appropriate comparative standards to determine the value of Perflubutane perfusion echocardiography relative to nuclear perfusion imaging. These standards are coronary angiography, nuclear perfusion imaging, and patient outcome.
Sonazoid is taken up by healthy Kupffer cells in the liver and spleen, but break down in high amplitude ultrasound imaging modes such as color Doppler imaging. The bubble rupture produces a transient pressure wave, which results in a characteristic mosaic color pattern from tissues containing the microbubbles (induced acoustic emission). Liver tumors without Kupffer cells will not display the mosaic pattern and can therefore be identified easily.
US-Technology Information Portal; Sonazoid. Available from, as of March 3, 2016: https://www.us-tip.com/serv1.php?type=db1&dbs=Sonazoid
...It is proposed that the intestinal and hepatic lesions in rats and mice after a single intravenous injection of gas-carrier contrast agents are caused by a common mechanism: intravascular growth of gas-carrier agents in tissues with gas supersaturation, as occurs in the cecal wall of rats and mice. In this particular environment the growing gas bubbles cause ischemia and necrosis in the cecal and colonic wall and liver. This proposed mechanism of action is consistent with the absence of clinical reports indicative of intestinal and/or hepatic lesions in humans after administration of gas-carrier contrast agents.
PMID:12729716 Dirven HA et al; Toxicol Appl Pharmacol 188 (3): 165-75 (2003)


