



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
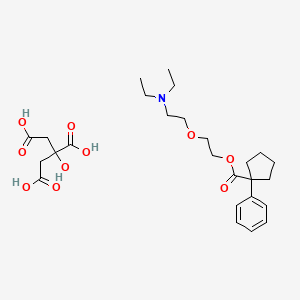

1. 2-(2-diethylaminoethoxy)ethyl 1-phenylcyclopentyl-1-carboxylate
2. Carbetapentane
3. Carbetapentane 1,5-napthalenedisulfonate (2:1)
4. Carbetapentane 2,6-napthalenedisulfonate (2:1)
5. Carbetapentane Tannate
6. Pentoxyverine
1. Pentoxyverine Citrate
2. 23142-01-0
3. Loucarbate
4. Toclase
5. Pentoxiverine Citrate
6. Carbetapentane (citrate)
7. Carbetapentane Citrate Salt
8. 4sh0mfj5hj
9. Nsc-757833
10. Pentoxyverine Hydrogen Citrate
11. Ethanol, 2-(2-(diethylamino)ethoxy)-, 1-phenylcyclopentanecarboxylate (ester) Citrate
12. Toclase Citrate
13. 2-(2-(diethylamino)ethoxy)ethyl 1-phenylcyclopentanecarboxylate Citrate (1:1)
14. Cyclopentanecarboxylic Acid, 1-phenyl-, 2-(2-(diethylamino)ethoxy)ethyl Ester, Citrate (1:1)
15. Carbetapentone Citrate
16. 2-[2-(diethylamino)ethoxy]ethyl 1-phenylcyclopentane-1-carboxylate Citrate
17. Carbetapentane Citrate [nf]
18. Sr-01000075435
19. Einecs 245-449-5
20. Unii-4sh0mfj5hj
21. Astomatop (tn)
22. 2-(2-(diethylamino)ethoxy)ethyl 1-phenylcyclopentanecarboxylate 2-hydroxypropane-1,2,3-tricarboxylate
23. Prestwick_748
24. Caretapentane Citrate
25. Mfcd00055697
26. Lopac-c-4662
27. Pentoxyverine Citrate,(s)
28. 2-(2(diethylamino)ethoxy)ethyl 1-phenyl-cyclopentanecarboxylate Citrate (1:1)
29. 2-(2-(diethylamino)ethoxy)ethyl 1-phenylcyclopentyl-1-carboxylate Dihydrogen Citrate
30. Mls000859895
31. Mls002207180
32. Schembl160096
33. Spectrum1501129
34. Pentoxyverine Citrate (jp17)
35. Chembl1256696
36. Chebi:31352
37. Hms501b18
38. Dtxsid10177717
39. Hms1569m15
40. Hms1921h13
41. Hms2092f03
42. Hms2096m15
43. Hms2233b06
44. Hms3260p08
45. Hms3266a18
46. Hms3370a07
47. Hms3411m11
48. Hms3675m11
49. Hms3713m15
50. Pharmakon1600-01501129
51. Carbetapentane Citrate [mi]
52. Pentoxyverine Citrate [jan]
53. Bcp13538
54. Ex-a4861
55. Hy-b1055
56. Tox21_500313
57. 2-(2-diethylaminoethoxy)ethyl 1-phenylcyclopentanecarboxylate Citrate
58. Ccg-39029
59. Nsc757833
60. Carbetapentane Citrate [vandf]
61. Pentoxyverine Citrate [mart.]
62. Akos015899622
63. Pentoxyverine Citrate [who-dd]
64. Cs-4577
65. Lp00313
66. Nsc 757833
67. 1-phenyl-cyclopentanecarboxylic Acid 2-[2-(diethylamino)ethoxy]ethyl Ester Citrate
68. Ncgc00016128-01
69. Ncgc00016128-02
70. Ncgc00016128-03
71. Ncgc00016128-04
72. Ncgc00016128-05
73. Ncgc00017090-01
74. Ncgc00093760-01
75. Ncgc00093760-02
76. Ncgc00093760-03
77. Ncgc00260998-01
78. Ac-19682
79. Ac-36723
80. As-35372
81. Bp166189
82. Diethyl(2-(2-((1-phenylcyclopentyl)formyloxy)ethoxy)ethyl)ammoniumdihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
83. Cas-23142-01-0
84. Db-046085
85. Eu-0100313
86. Ft-0630493
87. C 4662
88. D01260
89. 077p236
90. A839013
91. Pentoxyverine Hydrogen Citrate [ep Monograph]
92. Sr-01000075435-1
93. Sr-01000075435-3
94. Sr-01000075435-5
95. Sr-01000075435-7
96. Sr-01000075435-8
97. W-107426
98. Q27260431
99. Sr-01000075435-11
100. 2-(2-diethylaminoethoxy)ethyl 1-phenylcyclopentane Carboxylate Citrate
101. 1-phenylcyclopentanecarboxylic Acid 2-(2-diethylaminoethoxy)ethyl Ester Citrate
102. 2-[2-(diethylamino)ethoxy]ethyl 1-phenylcyclopentane-1-carboxylate; 2-oxidanylpropane-1,2,3-tricarboxylic Acid
103. 2-hydroxypropane-1,2,3-tricarboxylic Acid; 1-phenyl-1-cyclopentanecarboxylic Acid 2-[2-(diethylamino)ethoxy]ethyl Ester
| Molecular Weight | 525.6 g/mol |
|---|---|
| Molecular Formula | C26H39NO10 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 16 |
| Exact Mass | 525.25739644 g/mol |
| Monoisotopic Mass | 525.25739644 g/mol |
| Topological Polar Surface Area | 171 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 583 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)


