
Synopsis
Synopsis
0
JDMF
0
VMF
0
Australia
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
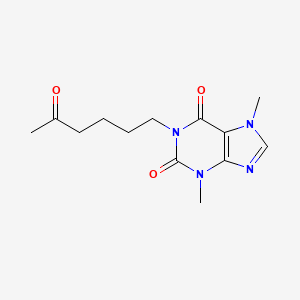

1. Agapurin
2. Bl 191
3. Bl-191
4. Bl191
5. Oxpentifylline
6. Pentoxil
7. Torental
8. Trental
1. Trental
2. 6493-05-6
3. Oxpentifylline
4. Pentoxifyllin
5. Torental
6. Pentoxyphylline
7. Dimethyloxohexylxanthine
8. Pentoxiphyllium
9. Vazofirin
10. 3,7-dimethyl-1-(5-oxohexyl)xanthine
11. Pentoxiphyllin
12. Pentoxiphylline
13. Rentylin
14. 1-(5-oxohexyl)theobromine
15. Bl 191
16. Bl-191
17. 1h-purine-2,6-dione, 3,7-dihydro-3,7-dimethyl-1-(5-oxohexyl)-
18. 3,7-dimethyl-1-(5-oxohexyl)purine-2,6-dione
19. Pentoxyphyllin
20. 3,7-dihydro-3,7-dimethyl-1-(5-oxohexyl)-1h-purine-2,6-dione
21. 3,7-dimethyl-1-(5-oxohexyl)-3,7-dihydro-1h-purine-2,6-dione
22. Oxypentifylline
23. Pentoxyfilline
24. 1-(5-oxohexyl)-3,7-dimethylxanthine
25. Theobromine, 1-(5-oxohexyl)-
26. C04ad03
27. Sd6qct3tsu
28. 3,7-dimethyl-1-(5-oxohexyl)-1h-purine-2,6(3h,7h)-dione
29. Nsc637086
30. Chembl628
31. Nsc 637086
32. Nsc-637086
33. Nsc-758481
34. 3,7-dimethyl-1-(5-oxohexyl)-1h,3h-purin-2,6-dione
35. Mls000079026
36. Chebi:7986
37. Pentoxyfylline
38. Azupentat
39. 1,2,3,6-tetrahydro-3,7-dimethyl-1-(5-oxohexyl)-2,6-purindion
40. 3,7-dimethyl-1-(5-oxohexyl)-2,3,6,7-tetrahydro-1h-purine-2,6-dione
41. Eht 0202
42. Ncgc00015801-02
43. Durapental
44. Smr000035998
45. Dsstox_cid_3437
46. Dsstox_rid_77028
47. Dsstox_gsid_23437
48. Pnx
49. Pentoxifyllinum
50. Pentoxifilina
51. Hemovas
52. Ralofect
53. Vasofirin
54. Ikomio
55. Agapurin Retard
56. Pentoxifilina [inn-spanish]
57. Pentoxifyllinum [inn-latin]
58. Pentoxil (tn)
59. Trental (tn)
60. Cas-6493-05-6
61. Eht0201
62. Sr-01000075641
63. Mfcd00063379
64. 3,7-dimethyl-1-(5-oxohexyl)xantine
65. 3,7-dimethyl-1-(5-oxohexyl) Xantine
66. Pentoxyifylline
67. Pentopak
68. Ccris 6832
69. Pentoxifylline (jan/usp/inn)
70. 3arr
71. 3aru
72. 3tvx
73. Eht-201
74. Ptx;oxpentifylline
75. Eht-0201
76. Prestwick_608
77. Einecs 229-374-5
78. Pentoxifylline,(s)
79. Brn 0558929
80. Spectrum_001444
81. 2a3c
82. Unii-sd6qct3tsu
83. Opera_id_1800
84. Prestwick0_000196
85. Prestwick1_000196
86. Prestwick2_000196
87. Prestwick3_000196
88. Spectrum2_001181
89. Spectrum3_001820
90. Spectrum4_000227
91. Spectrum5_001161
92. Lopac-p-1784
93. P 1784
94. Pentoxifylline [mi]
95. Pentoxifylline [inn]
96. Pentoxifylline [jan]
97. Lopac0_000936
98. Schembl34039
99. Bspbio_000151
100. Bspbio_003439
101. Kbiogr_000893
102. Kbioss_001924
103. Pentoxifylline [usan]
104. Mls000758298
105. Mls001201764
106. Mls001424051
107. Bidd:gt0174
108. Divk1c_000729
109. Pentoxifylline [vandf]
110. Spectrum1503611
111. Spbio_001221
112. Spbio_002072
113. Pentoxifylline [mart.]
114. Bpbio1_000167
115. Gtpl7095
116. 1-(3-carboxypropyl)-3,7-
117. Pentoxifylline [usp-rs]
118. Pentoxifylline [who-dd]
119. Dtxsid7023437
120. Bdbm10850
121. Hms502e11
122. Kbio1_000729
123. Kbio2_001924
124. Kbio2_004492
125. Kbio2_007060
126. Kbio3_002942
127. Ninds_000729
128. Hms1568h13
129. Hms1922e16
130. Hms2051n06
131. Hms2090h13
132. Hms2093g21
133. Hms2095h13
134. Hms2235c16
135. Hms3262l14
136. Hms3370d09
137. Hms3393n06
138. Hms3712h13
139. Pharmakon1600-01503611
140. Bcp29306
141. Hy-b0715
142. Zinc1530776
143. Pentoxifylline [orange Book]
144. Tox21_110223
145. Tox21_500936
146. Bbl016497
147. Ccg-36382
148. Nsc758481
149. Pdsp1_001015
150. Pdsp2_000999
151. Pentoxifylline [ep Monograph]
152. Stk177321
153. Pentoxifylline [usp Monograph]
154. Akos000541484
155. Tox21_110223_1
156. Ac-8381
157. Db00806
158. Lp00936
159. Nc00255
160. Sdccgsbi-0050910.p004
161. 3,7-dimethyl-1-(5-oxohexyl)-xanthine
162. Cas-1677687
163. Idi1_000729
164. Ncgc00015801-01
165. Ncgc00015801-03
166. Ncgc00015801-04
167. Ncgc00015801-05
168. Ncgc00015801-06
169. Ncgc00015801-07
170. Ncgc00015801-08
171. Ncgc00015801-09
172. Ncgc00015801-10
173. Ncgc00015801-12
174. Ncgc00015801-17
175. Ncgc00015801-21
176. Ncgc00067069-02
177. Ncgc00067069-03
178. Ncgc00067069-04
179. Ncgc00067069-05
180. Ncgc00178062-01
181. Ncgc00178062-02
182. Ncgc00261621-01
183. As-13662
184. Pentoxifylline [usan:usp:inn:ban:jan]
185. Sbi-0050910.p003
186. 1-(5-oxohexyl)theobromine (pentoxifylline)
187. Ab00052363
188. Eu-0100936
189. Ft-0603570
190. Ft-0657886
191. Ft-0673610
192. P2050
193. S4345
194. Sw196777-4
195. C07424
196. D00501
197. D70138
198. Ab00052363-17
199. Ab00052363_18
200. Ab00052363_21
201. 5-26-14-00081 (beilstein Handbook Reference)
202. Q416331
203. 3,7-dimethyl-1-(5-oxohexyl)-1h-purine-2,6-dione
204. Ptx; Oxpentifylline; Bl191; Bl 191; Bl-191
205. Sr-01000075641-1
206. Sr-01000075641-4
207. Sr-01000075641-7
208. Sr-01000075641-9
209. Brd-k57569181-001-05-1
210. Brd-k57569181-001-16-8
211. 3,7-dimethyl-1-(5-oxohexyl)-3,7-dihydropurine-2,6-dione
212. 3,7-dimethyl-1-(5-oxohexyl)-3,7-dihydro-1h-purine-2,6-dione #
213. Px
| Molecular Weight | 278.31 g/mol |
|---|---|
| Molecular Formula | C13H18N4O3 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 278.13789045 g/mol |
| Monoisotopic Mass | 278.13789045 g/mol |
| Topological Polar Surface Area | 75.5 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 426 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Pentoxifylline |
| PubMed Health | Pentoxifylline (By mouth) |
| Drug Classes | Hemorheologic |
| Drug Label | Each extended-release tablet, for oral administration, contains 400 mg of pentoxifylline and the following inactive ingredients: D&C Red #30 Aluminum Lake, FD&C Blue #2 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, hydroxyethyl cellulose, hypro |
| Active Ingredient | Pentoxifylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Apotex; Valeant Bermuda; Pliva; Mylan; Impax Labs |
| 2 of 4 | |
|---|---|
| Drug Name | Pentoxil |
| Drug Label | Pentoxil (Pentoxifylline Extended-release Tablets, USP) for oral administration contain 400 mg of the active drug and the following inactive ingredients: D&C Red No. 27 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, hypromellose USP, magnesium stea |
| Active Ingredient | Pentoxifylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Upsher Smith |
| 3 of 4 | |
|---|---|
| Drug Name | Pentoxifylline |
| PubMed Health | Pentoxifylline (By mouth) |
| Drug Classes | Hemorheologic |
| Drug Label | Each extended-release tablet, for oral administration, contains 400 mg of pentoxifylline and the following inactive ingredients: D&C Red #30 Aluminum Lake, FD&C Blue #2 Aluminum Lake, FD&C Yellow #6 Aluminum Lake, hydroxyethyl cellulose, hypro |
| Active Ingredient | Pentoxifylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Apotex; Valeant Bermuda; Pliva; Mylan; Impax Labs |
| 4 of 4 | |
|---|---|
| Drug Name | Pentoxil |
| Drug Label | Pentoxil (Pentoxifylline Extended-release Tablets, USP) for oral administration contain 400 mg of the active drug and the following inactive ingredients: D&C Red No. 27 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, hypromellose USP, magnesium stea |
| Active Ingredient | Pentoxifylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Upsher Smith |
Pentoxifylline is indicated for the treatment of intermittent claudication in patients with chronic occlusive arterial disease. Pentoxifylline may improve limb function and reduce symptoms but cannot replace other therapies such as surgical bypass or removal of vascular obstructions.
FDA Label
Investigated for use/treatment in alzheimer's disease and neurologic disorders.
Pentoxifylline, a synthetic dimethylxanthine derivative structurally related to [theophylline] and [caffeine], exhibits hemorheological, anti-oxidative, and anti-inflammatory properties and is traditionally indicated in the treatment of peripheral arterial disease (PAD). In PAD patients with concurrent cerebrovascular and coronary artery diseases, pentoxifylline treatment has occasionally been associated with angina, arrhythmia, and hypotension. Concurrent use with [warfarin] should be associated with more frequent monitoring of prothrombin times. Also, patients with risk factors complicated by hemorrhages, such as retinal bleeding, peptic ulceration, and recent surgery, should be monitored periodically for bleeding signs.
Phosphodiesterase Inhibitors
Compounds which inhibit or antagonize the biosynthesis or actions of phosphodiesterases. (See all compounds classified as Phosphodiesterase Inhibitors.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Free Radical Scavengers
Substances that eliminate free radicals. Among other effects, they protect PANCREATIC ISLETS against damage by CYTOKINES and prevent myocardial and pulmonary REPERFUSION INJURY. (See all compounds classified as Free Radical Scavengers.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Radiation-Protective Agents
Drugs used to protect against ionizing radiation. They are usually of interest for use in radiation therapy but have been considered for other purposes, e.g. military. (See all compounds classified as Radiation-Protective Agents.)
C04AD03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C04 - Peripheral vasodilators
C04A - Peripheral vasodilators
C04AD - Purine derivatives
C04AD03 - Pentoxifylline
Absorption
Oral pentoxifylline (PTX) is almost completely absorbed but has low bioavailability of 20-30% due to extensive first-pass metabolism; three of the seven known metabolites, M1, M4, and M5 are present in plasma and appear soon after dosing. Single oral doses of 100, 200, and 400 mg of pentoxifylline in healthy males produced a mean tmax of 0.29-0.41 h, a mean Cmax of 272-1607 ng/mL, and a mean AUC0- of 193-1229 ng\*h/mL; corresponding ranges for metabolites 1, 4, and 5 were 0.72-1.15, 114-2753, and 189-7057. Single administration of a 400 mg extended-release tablet resulted in a heightened tmax of 2.08 1.16 h, lowered Cmax of 55.33 22.04 ng/mL, and a comparable AUC0-t of 516 165 ng\*h/mL; all these parameters were increased in cirrhotic patients. Smoking was associated with a decrease in the Cmax and AUCsteady-state of metabolite M1 but did not dramatically affect the pharmacokinetic parameters of pentoxifylline or other measured metabolites. Renal impairment increases the mean Cmax, AUC, and ratio to parent compound AUC of metabolites M4 and M5, but has no significant effect on PTX or M1 pharmacokinetics. Finally, similar to cirrhotic patients, the Cmax and tmax of PTX and its metabolites are increased in patients with varying degrees of chronic heart failure. Overall, metabolites M1 and M5 exhibit plasma concentrations roughly five and eight times greater than PTX, respectively. PTX and M1 pharmacokinetics are approximately dose-dependent, while those of M5 are not. Food intake before PTX ingestion delays time to peak plasma concentrations but not overall absorption. Extended-release forms of PTX extend the tmax to between two and four hours but also serves to ameliorate peaks and troughs in plasma concentration over time.
Route of Elimination
Pentoxifylline is eliminated almost entirely in the urine and predominantly as M5, which accounts for between 57 and 65 percent of the administered dose. Smaller amounts of M4 are recovered, while M1 and the parent compound account for less than 1% of the recovered dose. The fecal route accounts for less than 4% of the administered dose.
Volume of Distribution
Pentoxifylline has a volume of distribution of 4.15 0.85 following a single intravenous 100 mg dose in healthy subjects.
Clearance
Pentoxifylline given as a single 100 mg intravenous infusion has a clearance of 3.62 0.75 L/h/kg in healthy subjects, which decreased to 1.44 0.46 L/h/kg in cirrhotic patients. In another study, the apparent clearance of either 300 or 600 mg of pentoxifylline given intravenously (median and range) was 4.2 (2.8-6.3) and 4.1 (2.3-4.6) L/min, respectively. It is important to note that, due to the reversible extra-hepatic metabolism of the parent compound and metabolite 1, the true clearance of pentoxifylline may be even higher than the measured values.
Pentoxifylline (PTX) metabolism is incompletely understood. There are seven known metabolites (M1 through M7), although only M1, M4, and M5 are detected in plasma at appreciable levels, following the general pattern M5 > M1 > PTX > M4. As PTX apparent clearance is higher than hepatic blood flow and the AUC ratio of M1 to PTX is not appreciably different in cirrhotic patients, it is clear that erythrocytes are the main site of PTX-M1 interconversion. However, the reaction likely occurs in the liver as well. PTX is reduced in an NADPH-dependent manner by unknown an unidentified carbonyl reductase to form either [lisofylline] (the (R)-M1 enantiomer) or (S)-M1; the reaction is stereoselective, producing (S)-M1 exclusively in liver cytosol, 85% (S)-M1 in liver microsomes, and a ratio of 0.010-0.025 R:S-M1 after IV or oral dosing in humans. Although both (R)- and (S)-M1 can be oxidized back into PTX, (R)-M1 can also give rise to M2 and M3 in liver microsomes. _In vitro_ studies suggest that CYP1A2 is at least partly responsible for the conversion of [lisofylline] ((R)-M1) back into PTX. Unlike the reversible oxidation/reduction of PTX and its M1 metabolites, M4 and M5 are formed via irreversible oxidation of PTX in the liver. Studies in mice recapitulating the PTX-ciprofloxacin drug reaction suggest that CYP1A2 is responsible for the formation of M6 from PTX and of M7 from M1, both through de-methylation at position 7. In general, metabolites M2, M3, and M6 are formed at very low levels in mammals.
Pentoxifylline is a known human metabolite of lisofylline.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Overall, pentoxifylline has an elimination half-life of between 0.39 and 0.84 hours, while its primary metabolites have elimination half-lives of between 0.96 and 1.61 hours.
Patients with peripheral arterial disease (PAD) may suffer from intermittent claudication, exertional leg pain that resolves upon rest, which is underscored by a complex etiology including vascular dysfunction (reduced limb perfusion, angiogenesis, and microcirculatory flow), systemic inflammation, and skeletal muscle dysfunction. Pentoxifylline (PTX), (3,7-dimethyl-1-(5-oxohexyl)-3,7-dihydro-1H-purine-2,6-dione) or 1-(5-oxohexyl)-3,7-dimethylxanthine, is a methyl-xanthine derivative that acts to lower blood viscosity by increasing erythrocyte flexibility, reducing plasma fibrinogen, inhibiting neutrophil activation, and suppressing erythrocyte/platelet aggregation; it also has antioxidant and anti-inflammatory effects. Although the precise mechanism of action has yet to be elucidated, numerous studies have suggested several effects of PTX. The classical interpretation of PTX's broad effects is due to its ability to act, _in vitro_, as a non-specific cyclic-3',5'-phosphodiesterase (PDE) inhibitor at millimolar concentrations; specifically, it has been proposed that inhibition of PDE type III and IV isozymes leads to elevated cyclic adenosine monophosphate (cAMP) levels, which mediate diverse downstream effects. This view has been challenged, specifically by observing those plasma concentrations of PTX in routine clinical use are typically only around 1M, far lower than those used to inhibit PDEs _in vitro_. Instead, several studies have suggested that PTX can modulate adenosine receptor function, specifically the G-coupled A2A receptor (A2AR). Whether PTX acts directly as an A2AR agonist is unclear, although it can clearly increase the response of A2AR to adenosine. A2AR activation activates adenylyl cyclase, which increases intracellular cAMP levels; this observation may explain PTX's ability to increase intracellular cAMP in a PDE-independent fashion. Elevated cAMP levels have numerous downstream effects. cAMP-mediated activation of protein kinase A (PKA) suppresses nuclear translocation of NF-B, which suppresses transcription of pro-inflammatory cytokines such as tumour necrosis factor (TNF-), interleukin-1 (IL-1), and IL-6 as well as TNF-induced molecules such as adhesion molecules (ICAM1 and VCAM1) and the C-reactive protein (CRP). PTX has also been shown to prevent the downstream phosphorylation of p38 MAPK and ERK, which are responsible for assembling the NADPH oxidase involved in the neutrophil oxidative burst. This effect is due to a PKA-independent decrease in Akt phosphorylation and a PKA-dependent decrease in phosphorylation of p38 MAPK and ERK. This transcriptional regulation at least partially explains the anti-inflammatory and anti-oxidative properties of PTX. Also, activated PKA can activate the cAMP response element-binding protein (CREB), which itself blocks SMAD-driven gene transcription, effectively disrupting transforming growth factor (TGF-1) signalling. This results in lower levels of fibrinogenic molecules such as collagens, fibronectin, connective tissue growth factor, and alpha-smooth muscle actin. Hence, disruption of TGF-1 signalling may explain the anti-fibrotic effects of PTX, including at least some of the decrease in blood viscosity. The picture is complicated by the observation that PTX metabolites M1, M4, and M5 have been shown to inhibit C5 Des Arg- and formyl-methionylleucylphenylalanine-induced superoxide production in neutrophils and M1 and M5 significantly contribute to PTX's observed hemorheological effects. Overall, PTX administration is associated with decreased pro-inflammatory molecules, an increase in anti-inflammatory molecules such as IL-10, and decreased production of fibrinogenic and cellular adhesion molecules.
EHT 0202 was discovered to play a role in protecting neurons in pharmacological models of neuronal cell death. ExonHit identified RNA isoforms produced by alterations of splicing specifically taking place in neurodegenerative disease models. These isoforms were identified using DATAS(TM), ExonHit's proprietary gene profiling technology. DATAS(TM), stands for Differential Analysis of Transcripts with Alternative Splicing.
 European CDMO and Gx manufacturer with 75 years of experience in delivering premium APIs to pharmaceutical partners worldwide.
European CDMO and Gx manufacturer with 75 years of experience in delivering premium APIs to pharmaceutical partners worldwide.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2014-12-16
Pay. Date : 2014-11-28
DMF Number : 20976
Submission : 2007-10-26
Status : Active
Type : II

Certificate Number : R1-CEP 2004-246 - Rev 05
Issue Date : 2023-01-27
Type : Chemical
Substance Number : 851
Status : Valid

NDC Package Code : 12658-0487
Start Marketing Date : 1998-07-20
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Wooshin Labotech Co., Ltd.
Registration Date : 2021-07-14
Registration Number : 20210714-209-J-1069
Manufacturer Name : Pharmaceutical Works Polpharma SA@[Starting Material Manufacturing Plant] Bakul Pharma Pvt. Ltd.
Manufacturer Address : 19, Pelplinska street, 83-200 Starogard Gdanski, Poland@[starting material manufacturing plant] Plot No. 6202, GIDC Ankleshwar 393 002, Dist. Bharuch, Gujarat, India

| Available Reg Filing : ASMF, CN |

 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
 Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.
Supriya Lifesciences is a cGMP-compliant API manufacturing organization with a leadership position across key & niche products.

Certificate Number : R0-CEP 2018-294 - Rev 00
Issue Date : 2021-01-12
Type : Chemical
Substance Number : 851
Status : Valid

Date of Issue : 2025-09-02
Valid Till : 2028-07-02
Written Confirmation Number : WC-0218
Address of the Firm :

NDC Package Code : 61281-3500
Start Marketing Date : 2016-11-02
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : ASMF |

 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 12562
Submission : 1997-06-26
Status : Active
Type : II

 Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.
Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.
 European CDMO and Gx manufacturer with 75 years of experience in delivering premium APIs to pharmaceutical partners worldwide.
European CDMO and Gx manufacturer with 75 years of experience in delivering premium APIs to pharmaceutical partners worldwide.
 European CDMO and Gx manufacturer with 75 years of experience in delivering premium APIs to pharmaceutical partners worldwide.
European CDMO and Gx manufacturer with 75 years of experience in delivering premium APIs to pharmaceutical partners worldwide.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
CAS Number : 10226-30-9
End Use API : Pentoxifylline
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

CAS Number : 10226-30-9
End Use API : Pentoxifylline
About The Company : Founded with a mission to transform strategic capital into specialty chemicals, Ami Group focuses on Agrochemicals, Cosmetics, and Polymers. Ami Organics Ltd. i...

CAS Number : 10226-30-9
End Use API : Pentoxifylline
About The Company : Chemclone Industries is India’s leading manufacturer of bromide derivatives, specialty chemicals, quaternary ammonium and phosphonium salts, APIs, and interme...

CAS Number : 10226-30-9
End Use API : Pentoxifylline
About The Company : Litmus Organics Pvt. Ltd., established in 2005, aims to be a leading chemical manufacturer in India. Equipped with the latest technology and testing facilities,...

CAS Number : 10226-30-9
End Use API : Pentoxifylline
About The Company : RR LIFESCIENCES manufactures products purely by organic synthesis to offer its customers a choice of products in their areas. Our facilities are equipped with a...

CAS Number : 10226-30-9
End Use API : Pentoxifylline
About The Company : Established in 2000, Shodhana Laboratories offers a wide range of APIs and intermediates and caters to the generic and custom requirements of some of the most p...

3,7-Dihydro-3,7-Dimethyl-1H-purine-2,6-Dione or Th...
CAS Number : 83-67-0
End Use API : Pentoxifylline
About The Company : Established in 2006, SPC Lifesciences is a truly knowledge driven and fully integrated pharmaceutical company creating global solutions. We are a dynamic chemic...

CAS Number : 10226-30-9
End Use API : Pentoxifylline
About The Company : Established in 2006, SPC Lifesciences is a truly knowledge driven and fully integrated pharmaceutical company creating global solutions. We are a dynamic chemic...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Italy
Brand Name : Trental
Dosage Form :
Dosage Strength : 30 Cpr Riv 400 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Italy
Brand Name : Trental
Dosage Form :
Dosage Strength : 30 Cpr Riv 600 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info :
Registration Country : Italy
Brand Name : Trental
Dosage Form :
Dosage Strength : 5 Ampoules Ev 5 Ml 100 Mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy
 Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Sanofi is a pioneer in Diabetes Solutions, Human Vaccines, Innovative Drugs, Consumer Healthcare, and the new Genzyme.
Regulatory Info : Withdrawn
Registration Country : Malta
Brand Name : Trental
Dosage Form : Modified Release Tablet
Dosage Strength : 400MG
Packaging :
Approval Date : 2005-10-27
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Denmark
Brand Name : Trental
Dosage Form : Prolonged-Release Tablet
Dosage Strength : 400mg
Packaging :
Approval Date : 23-01-2017
Application Number : 28105873016
Regulatory Info : Prescription
Registration Country : Denmark

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Hemovas
Dosage Form : Extended-Release Tablet
Dosage Strength : 600MG
Packaging :
Approval Date : 29-09-2000
Application Number : 63374
Regulatory Info : Authorized
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Moldova
Brand Name : Pentilin® retard
Dosage Form : Extended-Release Tablet
Dosage Strength : 400mg
Packaging :
Approval Date : 21-06-2019
Application Number :
Regulatory Info :
Registration Country : Moldova

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Estonia
Brand Name : Pentilin
Dosage Form : Tablet
Dosage Strength : 400mg
Packaging :
Approval Date :
Application Number :
Regulatory Info : Prescription
Registration Country : Estonia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Allowed
Registration Country : Switzerland
Brand Name : Pentoxi-Mepha
Dosage Form : Prolonged Release Tablet
Dosage Strength : 400mg
Packaging :
Approval Date : 30/06/1988
Application Number : 49324
Regulatory Info : Allowed
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Hungary
Brand Name : Chinotal
Dosage Form : Film-Coated Tablet
Dosage Strength : 400MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Hungary

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
13 Aug 2025
Reply
02 Sep 2024
Reply
22 Jul 2024
Reply
21 Jun 2024
Reply
17 Oct 2023
Reply
18 Jul 2023
Reply
01 Mar 2023
Reply
30 Mar 2022
Reply
01 Oct 2020
Reply
16 Mar 2020
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
50
PharmaCompass offers a list of Pentoxifylline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pentoxifylline manufacturer or Pentoxifylline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pentoxifylline manufacturer or Pentoxifylline supplier.
PharmaCompass also assists you with knowing the Pentoxifylline API Price utilized in the formulation of products. Pentoxifylline API Price is not always fixed or binding as the Pentoxifylline Price is obtained through a variety of data sources. The Pentoxifylline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pentoxifylline manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pentoxifylline, including repackagers and relabelers. The FDA regulates Pentoxifylline manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pentoxifylline API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pentoxifylline manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pentoxifylline supplier is an individual or a company that provides Pentoxifylline active pharmaceutical ingredient (API) or Pentoxifylline finished formulations upon request. The Pentoxifylline suppliers may include Pentoxifylline API manufacturers, exporters, distributors and traders.
click here to find a list of Pentoxifylline suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pentoxifylline DMF (Drug Master File) is a document detailing the whole manufacturing process of Pentoxifylline active pharmaceutical ingredient (API) in detail. Different forms of Pentoxifylline DMFs exist exist since differing nations have different regulations, such as Pentoxifylline USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pentoxifylline DMF submitted to regulatory agencies in the US is known as a USDMF. Pentoxifylline USDMF includes data on Pentoxifylline's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pentoxifylline USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pentoxifylline suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Pentoxifylline Drug Master File in Korea (Pentoxifylline KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Pentoxifylline. The MFDS reviews the Pentoxifylline KDMF as part of the drug registration process and uses the information provided in the Pentoxifylline KDMF to evaluate the safety and efficacy of the drug.
After submitting a Pentoxifylline KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Pentoxifylline API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Pentoxifylline suppliers with KDMF on PharmaCompass.
A Pentoxifylline CEP of the European Pharmacopoeia monograph is often referred to as a Pentoxifylline Certificate of Suitability (COS). The purpose of a Pentoxifylline CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Pentoxifylline EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Pentoxifylline to their clients by showing that a Pentoxifylline CEP has been issued for it. The manufacturer submits a Pentoxifylline CEP (COS) as part of the market authorization procedure, and it takes on the role of a Pentoxifylline CEP holder for the record. Additionally, the data presented in the Pentoxifylline CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Pentoxifylline DMF.
A Pentoxifylline CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Pentoxifylline CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Pentoxifylline suppliers with CEP (COS) on PharmaCompass.
A Pentoxifylline written confirmation (Pentoxifylline WC) is an official document issued by a regulatory agency to a Pentoxifylline manufacturer, verifying that the manufacturing facility of a Pentoxifylline active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Pentoxifylline APIs or Pentoxifylline finished pharmaceutical products to another nation, regulatory agencies frequently require a Pentoxifylline WC (written confirmation) as part of the regulatory process.
click here to find a list of Pentoxifylline suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pentoxifylline as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pentoxifylline API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pentoxifylline as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pentoxifylline and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pentoxifylline NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pentoxifylline suppliers with NDC on PharmaCompass.
Pentoxifylline Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pentoxifylline GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pentoxifylline GMP manufacturer or Pentoxifylline GMP API supplier for your needs.
A Pentoxifylline CoA (Certificate of Analysis) is a formal document that attests to Pentoxifylline's compliance with Pentoxifylline specifications and serves as a tool for batch-level quality control.
Pentoxifylline CoA mostly includes findings from lab analyses of a specific batch. For each Pentoxifylline CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pentoxifylline may be tested according to a variety of international standards, such as European Pharmacopoeia (Pentoxifylline EP), Pentoxifylline JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pentoxifylline USP).

