



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
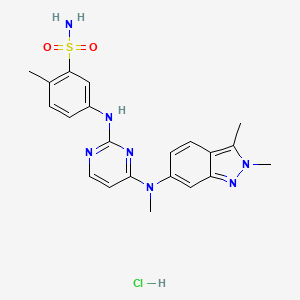

1. Gw 780604
2. Gw 786034b
3. Gw-780604
4. Gw-786034b
5. Gw780604
6. Gw786034b
7. Pazopanib
8. Votrient
1. 635702-64-6
2. Pazopanib Hcl
3. Votrient
4. Pazopanib (hydrochloride)
5. Armala
6. Unii-33y9anm545
7. Pazopanib Monohydrochloride
8. Pazopanib Hcl (gw786034 Hcl)
9. Gw786034b
10. Gw-786034b
11. Pazopanib (as Hydrochloride)
12. Gw786034 (hydrochloride)
13. 5-[[4-[(2,3-dimethylindazol-6-yl)-methylamino]pyrimidin-2-yl]amino]-2-methylbenzenesulfonamide;hydrochloride
14. Chebi:71217
15. 33y9anm545
16. S1035
17. 5-((4-((2,3-dimethyl-2h-indazol-6-yl)(methyl)amino)pyrimidin-2-yl)amino)-2-methylbenzenesulfonamide Hydrochloride
18. 5-((4-((2,3-dimethyl-2h-indazol-6-yl)methylamino)pyrimidin-2-yl)amino)-2-methylbenzenesulfonamide Monohydrochloride
19. 5-({4-[(2,3-dimethyl-2h-indazol-6-yl)(methyl)amino]pyrimidin-2-yl}amino)-2-methylbenzene-1-sulfonamide Hydrochloride
20. 5-{4-[(2,3-dimethyl-2h-indazol-6-yl)-methyl-amino]-pyrimidin-2-ylamino}-2-methyl-benzenesulfonamide Hydrochloride
21. Benzenesulfonamide, 5-((4-((2,3-dimethyl-2h-indazol-6-yl)methylamino)-2-pyrimidinyl)amino)-2-methyl-, Monohydrochloride
22. Patorma
23. Gw786034 Hcl
24. Votrient Hcl
25. Votrient (tn)
26. 5-({4-[(2,3-dimethyl-2h-indazol-6-yl)(methyl)amino]pyrimidin-2-yl}amino)-2-methylbenzenesulfonamide Hydrochloride
27. Pazopanib Hydrochloride [usan:jan]
28. Mls004774147
29. Schembl159487
30. Pazopanib Hydrochloride- Bio-x
31. Pazopanib Hydrochloride [usan]
32. Chembl1201733
33. Dtxsid70212956
34. Ex-a956
35. Pazopanib Hcl (gw786034 )
36. Bcp02203
37. Pazopanib Hydrochloride (jan/usan)
38. Pazopanib Hydrochloride [mi]
39. Mfcd12546138
40. Nsc737754
41. Pazopanib Hydrochloride [jan]
42. Akos015958593
43. Bcp9001052
44. Ccg-269485
45. Cs-0126
46. Gsk-786034
47. Ks-1458
48. Nsc-737754
49. Pazopanib Hydrochloride [mart.]
50. Pb31655
51. Sb10367
52. Pazopanib Hydrochloride [who-dd]
53. Ac-24726
54. Bp166746
55. Hy-12009
56. Smr003500790
57. Am20090655
58. Ft-0687642
59. P2243
60. Pazopanib Hydrochloride [orange Book]
61. Sw218082-2
62. Ec-000.2347
63. D05380
64. A834417
65. Q27888072
66. 5-({4-[(2,3-dimethyl-2h-indazol-6-yl)(methyl)amino]-2-pyrimidinyl}amino)-2-methylbenzenesulfonamide Hydrochloride
67. 5-(4-((2,3-dimethyl-2h-indazol-6-yl)(methyl)amino)pyrimidin-2-ylamino)-2-methylbenzenesulfonamide Hydrochloride
68. 5-(4-((2,3-dimethyl-2h-indazol-6-yl)(methyl)amino)pyrimidin-2-ylamino)-2-methylbenzenesulfonamide Hydrochloride;5-[[4-[(2,3-dimethylindazol-6-yl)-methyl-amino]pyrimidin-2-yl]amino]-2-methyl-benzenesulfonamide Hydrochloride
69. 5-[[4-[(2,3-dimethylindazol-6-yl)-methylamino]pyrimidin-2-yl]amino]-2-methylbenzenesulfonamide Hydrochloride
70. Benzenesulfonamide, 5-[[4-[(2,3-dimethyl-2h-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-, Hydrochloride (1:1)
| Molecular Weight | 474.0 g/mol |
|---|---|
| Molecular Formula | C21H24ClN7O2S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 473.1400719 g/mol |
| Monoisotopic Mass | 473.1400719 g/mol |
| Topological Polar Surface Area | 127 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 717 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Votrient |
| PubMed Health | Pazopanib (By mouth) |
| Drug Classes | Antineoplastic Agent, Immunological Agent |
| Active Ingredient | Pazopanib hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 200mg base; eq 400mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 2 | |
|---|---|
| Drug Name | Votrient |
| PubMed Health | Pazopanib (By mouth) |
| Drug Classes | Antineoplastic Agent, Immunological Agent |
| Active Ingredient | Pazopanib hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 200mg base; eq 400mg base |
| Market Status | Prescription |
| Company | Glaxosmithkline |
* Renal-cell carcinoma (RCC):
Votrient is indicated in adults for the first-line treatment of advanced renal-cell carcinoma (RCC) and for patients who have received prior cytokine therapy for advanced disease.
* Soft-tissue sarcoma (STS):
Votrient is indicated for the treatment of adult patients with selective subtypes of advanced soft-tissue sarcoma (STS) who have received prior chemotherapy for metastatic disease or who have progressed within 12 months after (neo)adjuvant therapy.
Efficacy and safety have only been established in certain STS histological tumour subtypes.
L01XE11








