


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
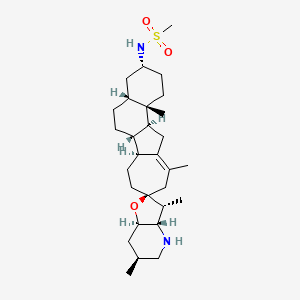

1. Ipi-926
1. Patidegib
2. Ipi-926
3. 1037210-93-7
4. Ipi 926
5. Ip9 Free Base
6. Fin-5
7. Jt96fpu35x
8. Ipi-926 Free Base
9. Chembl538867
10. Ip-9
11. Patidegib (usan)
12. Patidegib [usan]
13. N-[(3r,3'r,3'as,4ar,6's,6ar,6bs,7'ar,9s,12as,12bs)-3',6',11,12b-tetramethylspiro[1,2,3,4,4a,5,6,6a,6b,7,8,10,12,12a-tetradecahydronaphtho[2,1-a]azulene-9,2'-3a,4,5,6,7,7a-hexahydro-3h-furo[3,2-b]pyridine]-3-yl]methanesulfonamide
14. N-((2s,3r,3as,3'r,4a'r,6s,6a'r,6b's,7ar,12a's,12b's)-3,6,11',12b'-tetramethyl-2',3a,3',4,4',4a',5,5',6,6',6a',6b',7,7a,7',8',10',12',12a',12b'-icosahydro-1'h,3h-spiro[furo[3,2-b]pyridine-2,9'-naphtho[2,1-a]azulen]-3'-yl)methanesulfonamide
15. Patidegib [usan:inn]
16. Unii-jt96fpu35x
17. Saridegib [rescinded Usan]
18. Patidegib [inn]
19. Patidegib [who-dd]
20. Schembl421999
21. Gtpl8198
22. Dtxsid40146032
23. Chebi:177425
24. Saridegib (ipi-926; Patidegib)
25. Who 9619
26. Bdbm50293788
27. Zinc43197072
28. Db12655
29. Hy-16587
30. Cs-0007501
31. D10324
32. Q15426668
33. Methanesulfonamide, N-((2s,3r,3'r,3as,4'ar,6s,6'ar,6'bs,7ar,12'as,12'bs)- 2',3',3a,4,4',4'a,5,5',6,6',6'a,6'b,7,7',7a,8',10',12',12'a,12'b-eicosahydro-3,6,11',12'b-tetramethylspiro(furo(3,2-b)pyridine-2(3h),9'(1'h)-naphth(2,1-a)azulen)-3'-yl)-
34. Methanesulfonamide, N-((2s,3r,3'r,3as,4'ar,6s,6'ar,6'bs,7ar,12'as,12'bs)-2',3',3a,4,4',4'a,5,5',6,6',6'a,6'b,7,7',7a,8',10',12',12'a,12'b-eicosahydro-3,6,11',12'b-tetramethylspiro(furo(3,2-b)pyridine-2(3h),9'(1'h)-naphth(2,1-a)azulen)-3'-yl)-
35. N-((2s,3r,3as,3''r,4a''r,6s,6a''r,6b''s,7ar,12a''s,12b''s)-3,6,11'',12b''-tetramethyl-2'',3a,3'',4,4'',4a'',5,5'',6,6'',6a'',6b'',7,7a,7'',8'',10'',12'',12a'',12b''-icosahydro-1''h,3h-spiro[furo[3,2-b]pyridine-2,9''-naphtho[2,1-a]azulene]-3''-yl)methanesulfonamide
36. N-[(3r,3'r,3'as,4ar,6's,6ar,6bs,7'ar,9s,12as,12bs)-3',6',11,12b-tetramethylspiro[1,2,3,4,4a,5,6,6a,6b,7,8,10,12,12a-tetradecahydronaphtho[2,1-a]azulene-9,2'-3a,4,5,6,7,7a-hexahydro-3h-uro[3,2-b]pyridine]-3-yl]methanesulonamide
| Molecular Weight | 504.8 g/mol |
|---|---|
| Molecular Formula | C29H48N2O3S |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 504.33856457 g/mol |
| Monoisotopic Mass | 504.33856457 g/mol |
| Topological Polar Surface Area | 75.8 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 988 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 11 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of naevoid basal-cell carcinoma syndrome (Gorlin syndrome)


