
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
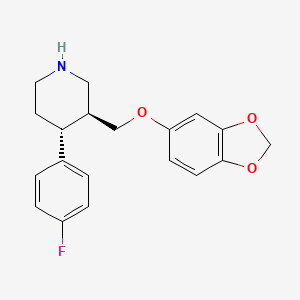

1. Aropax
2. Brl 29060
3. Brl-29060
4. Brl29060
5. Fg 7051
6. Fg-7051
7. Fg7051
8. Paroxetine Acetate
9. Paroxetine Hydrochloride
10. Paroxetine Hydrochloride Anhydrous
11. Paroxetine Hydrochloride Hemihydrate
12. Paroxetine Hydrochloride, Hemihydrate
13. Paroxetine Maleate
14. Paroxetine, Cis-(+)-isomer
15. Paroxetine, Cis-(-)-isomer
16. Paroxetine, Trans-(+)-isomer
17. Paxil
18. Seroxat
1. 61869-08-7
2. Paxil
3. Frosinor
4. Paxil Cr
5. Casbol
6. Motivan
7. Paxpar
8. Seroxat
9. Paroxetina
10. Paroxetinum
11. Aropax
12. Paroxetinum [inn-latin]
13. Paroxetina [inn-spanish]
14. Brl 29060
15. Paxetil
16. Brl-29060
17. (-)-paroxetine
18. Fg 7051
19. Fg-7051
20. (3s,4r)-3-((benzo[d][1,3]dioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidine
21. Arketis
22. Besitram
23. Daparox
24. Parogen
25. Xetanor
26. Arotin
27. (3s,4r)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine
28. (-)-(3s,4r)-4-(p-fluorophenyl)-3-((3,4-(methylenedioxy)phenoxy)methyl)piperidine
29. [3h]paroxetine
30. (+/-)-paroxetine
31. (-)3s,4r-paroxetine
32. (3s,4r)-3-(1,3-benzodioxol-5-yloxymethyl)-4-(4-fluorophenyl)piperidine
33. Paroxetine, (+/-)-
34. Chembl490
35. Paroxetine, Trans-(+/-)-
36. Piperidine, 3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-, (3s,4r)-
37. 32q7tw8bx7
38. Chebi:7936
39. 41vrh5220h
40. (-)-(3s,4r)-4-(p-fluorophenyl)-3-((3,4-methylenedioxy)phenoxy)methyl)piperidine
41. Piperidine, 3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-, (3s-trans)-
42. Dsstox_cid_3425
43. (3s,4r)-3-[(2h-1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)piperidine
44. (3s-trans)-3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidine
45. 110429-35-1
46. Paroxetine (tn)
47. Dsstox_rid_77022
48. Dsstox_gsid_23425
49. Paroxetine [usan:inn:ban]
50. Chembl1708
51. Piperidine, 3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-, (3r,4s)-rel-
52. Piperidine, 3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-, Trans-
53. Piperidine, 3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)-, Trans-(+/-)-
54. Rel-(3r,4s)-3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidine
55. Paroxetine Hcl Hemihydrate
56. 130855-15-1
57. Cas-61869-08-7
58. Paroxetine (usp/inn)
59. Unii-41vrh5220h
60. 3h-paroxetine
61. Paroxetine.hcl
62. (3s,4r)-3-((1,3-benzodioxol-5-yloxy)methyl)-4-(4-fluorophenyl)piperidine
63. Piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, (3s,4r)-
64. Piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-, (3s-trans)-
65. Ncgc00182968-01
66. 8pr
67. Nnc-20-7051
68. Spectrum_001752
69. Paroxetine [mi]
70. Specplus_000788
71. Paroxetine [inn]
72. Prestwick3_000851
73. Spectrum5_001665
74. Paroxetine [usan]
75. Paroxetine [vandf]
76. Paroxetine [mart.]
77. Paroxetine [who-dd]
78. Unii-32q7tw8bx7
79. Schembl27799
80. Bspbio_000861
81. Kbioss_002232
82. Bidd:gt0673
83. Divk1c_006884
84. Bpbio1_000949
85. Gtpl4790
86. Dtxsid3023425
87. Bdbm22416
88. Hsdb 7175
89. Kbio1_001828
90. Kbio2_002232
91. Kbio2_004800
92. Kbio2_007368
93. Hms2090h05
94. Zinc527386
95. Tox21_113123
96. Bdbm50331515
97. (-)-trans-4-(4-fluorophenyl)-3-(3,4-methylenedioxyphenoxymethyl)piperidine
98. Akos015888636
99. Tox21_113123_1
100. Ac-8185
101. Db00715
102. Sdccgsbi-0051908.p003
103. (-)-trans-4-(p-fluorophenyl)-3-[[3,4-(methylenedioxy)phenoxy]methyl]-piperidine
104. (3s-trans)-3-[(1,3-benzodioxol-5-yl-oxy)methyl]-4-(4-fluorophenyl)piperidine
105. Ncgc00025355-02
106. Ncgc00025355-03
107. Ncgc00025355-04
108. Ncgc00025355-05
109. Ncgc00025355-06
110. Ncgc00025355-07
111. Ncgc00025355-08
112. Ncgc00025355-09
113. Ncgc00025355-12
114. Ncgc00025355-22
115. Sbi-0051908.p002
116. Hy-122272
117. Ab00514724
118. Cs-0083299
119. C07415
120. D02362
121. Ab00053704-21
122. Ab00053704_22
123. Ab00053704_23
124. Paroxetine Hydrochloride Anhydrous Ep Impurity E
125. 869p087
126. Paroxetine Hydrochloride (anhydrous Or Hemihydrate)
127. Q408471
128. Brd-k37991163-003-02-7
129. Brd-k37991163-050-05-1
130. (-)-trans-4-(p-fluorophenyl)-3-((3,4-(methylenedioxy)phenoxy)methyl)piperidine
131. Piperidine, 3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-,(3s,4r)-
132. Cis-paroxetine; Paroxetine Usp Related Compound D;[(3s,4r)-4-(p-fluorophenyl)-3-piperidyl](1,3-dioxa-5-indanyloxy)methane
| Molecular Weight | 329.4 g/mol |
|---|---|
| Molecular Formula | C19H20FNO3 |
| XLogP3 | 3.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 329.14272166 g/mol |
| Monoisotopic Mass | 329.14272166 g/mol |
| Topological Polar Surface Area | 39.7 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 402 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Paxil |
| PubMed Health | Paroxetine (By mouth) |
| Drug Classes | Antidepressant, Central Nervous System Agent |
| Drug Label | DESCRIPTIONPAXIL CR (paroxetine hydrochloride) is an orally administered psychotropic drug with a chemical structure unrelated to other selective serotonin reuptake inhibitors or to tricyclic, tetracyclic, or other available antidepressant or antipan... |
| Active Ingredient | Paroxetine hydrochloride |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | eq 20mg base; eq 40mg base; eq 10mg base/5ml; eq 30mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Apotex Technologies |
| 2 of 6 | |
|---|---|
| Drug Name | Paxil cr |
| Drug Label | PAXIL CR (paroxetine hydrochloride) is an orally administered psychotropic drug with a chemical structure unrelated to other selective serotonin reuptake inhibitors or to tricyclic, tetracyclic, or other available antidepressant or antipanic agents.... |
| Active Ingredient | Paroxetine hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 12.5mg base; eq 25mg base; eq 37.5mg base |
| Market Status | Prescription |
| Company | Apotex Technologies |
| 3 of 6 | |
|---|---|
| Drug Name | Pexeva |
| Active Ingredient | Paroxetine mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base; eq 40mg base; eq 30mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Noven Therap |
| 4 of 6 | |
|---|---|
| Drug Name | Paxil |
| PubMed Health | Paroxetine (By mouth) |
| Drug Classes | Antidepressant, Central Nervous System Agent |
| Drug Label | DESCRIPTIONPAXIL CR (paroxetine hydrochloride) is an orally administered psychotropic drug with a chemical structure unrelated to other selective serotonin reuptake inhibitors or to tricyclic, tetracyclic, or other available antidepressant or antipan... |
| Active Ingredient | Paroxetine hydrochloride |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | eq 20mg base; eq 40mg base; eq 10mg base/5ml; eq 30mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Apotex Technologies |
| 5 of 6 | |
|---|---|
| Drug Name | Paxil cr |
| Drug Label | PAXIL CR (paroxetine hydrochloride) is an orally administered psychotropic drug with a chemical structure unrelated to other selective serotonin reuptake inhibitors or to tricyclic, tetracyclic, or other available antidepressant or antipanic agents.... |
| Active Ingredient | Paroxetine hydrochloride |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | eq 12.5mg base; eq 25mg base; eq 37.5mg base |
| Market Status | Prescription |
| Company | Apotex Technologies |
| 6 of 6 | |
|---|---|
| Drug Name | Pexeva |
| Active Ingredient | Paroxetine mesylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base; eq 40mg base; eq 30mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Noven Therap |
Antidepressive Agents, Second-Generation; Serotonin Uptake Inhibitors
National Library of Medicine's Medical Subject Headings. Paroxetine. Online file (MeSH, 2015). Available from, as of May 1, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Paroxetine is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 18, 2015: https://clinicaltrials.gov/search/intervention=paroxetine
Paxil is indicated for the treatment of major depressive disorder. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Paxil (Paroxetine Hydrochloride Hemihydrate) Tablet, Film Coated; Paxil (Paroxetine Hydrochloride Hemihydrate) Suspension (Updated: July 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=584ace29-6e40-432f-950f-ab7e98653d32
Paxil is indicated for the treatment of obsessions and compulsions in patients with obsessive compulsive disorder (OCD) as defined in the DSM-IV. The obsessions or compulsions cause marked distress, are time-consuming, or significantly interfere with social or occupational functioning. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Paxil (Paroxetine Hydrochloride Hemihydrate) Tablet, Film Coated; Paxil (Paroxetine Hydrochloride Hemihydrate) Suspension (Updated: July 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=584ace29-6e40-432f-950f-ab7e98653d32
For more Therapeutic Uses (Complete) data for PAROXETINE (13 total), please visit the HSDB record page.
/BOXED WARNING/ SUICIDALITY AND ANTIDEPRESSANT DRUGS. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of Paxil or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Paxil is not approved for use in pediatric patients.
NIH; DailyMed. Current Medication Information for Paxil (Paroxetine Hydrochloride Hemihydrate) Tablet, Film Coated; Paxil (Paroxetine Hydrochloride Hemihydrate) Suspension (Updated: July 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=584ace29-6e40-432f-950f-ab7e98653d32
/BOXED WARNING/ WARNING: SUICIDAL THOUGHTS AND BEHAVIORS. Antidepressants, including selective serotonin reuptake inhibitors (SSRIs), have been shown to increase the risk of suicidal thoughts and behavior in pediatric and young adult patients when used to treat major depressive disorder and other psychiatric disorders. Because Brisdelle is an SSRI, monitor patients closely for worsening and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber.
NIH; DailyMed. Current Medication Information for Brisdelle (Paroxetine Mesylate) Capsule (Updated: December 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bf208751-e6d8-11e1-aff1-0800200c9a66
Somnolence, which appears to be dose related, is among the most common adverse effects of paroxetine, occurring in approximately 23% of depressed patients receiving the drug in short-term controlled clinical trials. Somnolence required discontinuance of therapy in about 2% of patients. Headache occurred in about 18 or 15% of patients receiving paroxetine in short- or long-term controlled clinical trials, respectively. In addition, migraine or vascular headache has been reported in up to 1% or less than 0.1% of paroxetine-treated patients, respectively. Asthenia, which also appears to be dose related,1 occurred in 15% of depressed patients receiving the drug in short-term controlled clinical trials and required discontinuance of therapy in about 2% of patients.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2348
Dizziness, which appears to be dose related, occurred in about 13% of patients receiving paroxetine in short-term controlled clinical trials. Insomnia occurred in about 13 or 8% of patients receiving the drug in short- or long-term controlled clinical trials, respectively. However, because insomnia is a symptom also associated with depression, relief of insomnia and improvement in sleep patterns may occur when clinical improvement in depression becomes apparent during antidepressant therapy. In clinical trials, less than 2% of patients discontinued paroxetine because of insomnia.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2348
For more Drug Warnings (Complete) data for PAROXETINE (41 total), please visit the HSDB record page.
Paroxetine is indicated for the management of depression, obsessive-compulsive disorder, panic disorder, social anxiety disorder, generalized anxiety disorder, posttraumatic stress disorder. One form of paroxetine, commercially known as Brisdelle, is used to manage mild to moderate vasomotor symptoms of menopause. Off-label, paroxetine may be used for the treatment of premature ejaculation or irritable bowel syndrome (IBS).
Paroxetine treats the symptoms of depression, various anxiety disorders, posttraumatic stress disorder, obsessive-compulsive disorder, and the vasomotor symptoms of menopause via the inhibition of serotonin reuptake. The onset of action of paroxetine is reported to be approximately 6 weeks. Due its serotonergic activity, paroxetine, like other SSRI drugs, may potentiate serotonin syndrome. This risk is especially high when monoamine oxidase (MAO) inhibitors are given within 2 weeks of paroxetine administration. Upon cessation of MAO inhibitors, a 2-week interval before paroxetine administration is recommended. Do not coadminister these agents.
Cytochrome P-450 CYP2D6 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2D6. (See all compounds classified as Cytochrome P-450 CYP2D6 Inhibitors.)
Selective Serotonin Reuptake Inhibitors
Compounds that specifically inhibit the reuptake of serotonin in the brain. (See all compounds classified as Selective Serotonin Reuptake Inhibitors.)
Antidepressive Agents, Second-Generation
A structurally and mechanistically diverse group of drugs that are not tricyclics or monoamine oxidase inhibitors. The most clinically important appear to act selectively on serotonergic systems, especially by inhibiting serotonin reuptake. (See all compounds classified as Antidepressive Agents, Second-Generation.)
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AB - Selective serotonin reuptake inhibitors
N06AB05 - Paroxetine
Absorption
Paroxetine is readily absorbed from the gastrointestinal tract. Due to the first-pass metabolism, the bioavailability ranges from 30-60%. Cmax is attained 2 to 8 hours after an oral dose. Mean Tmax is 4.3 hours in healthy patients. The steady-state concentration of paroxetine is achieved within 7 to 14 days of oral therapy. In a pharmacokinetic study, AUC in healthy patients was 574 ngh/mL and 1053 ngh/mL in those with moderate renal impairment.
Route of Elimination
About 2/3 of a single paroxetine dose is found to be excreted in the urine and the remainder is found to be excreted in feces. Almost all of the dose is eliminated as metabolites; 3% is found to be excreted as unchanged paroxetine. About 64% of a 30 mg oral dose was found excreted in the urine, with 2% as the parent drug and 62% appearing as metabolites. Approximately 36% of the dose was found to be eliminated in the feces primarily as metabolites and less than 1% as the parent compound.
Volume of Distribution
Paroxetine has a large volume of distribution and is found throughout the body, including in the central nervous system. Only 1% of the drug is found in the plasma. Paroxetine is found in the breast milk at concentrations similar to the concentrations found in plasma.
Clearance
The apparent oral clearance of paroxetine is 167 L/h. The clearance of paroxetine in patients with renal failure is significantly lower and dose adjustment may be required, despite the fact that it is mainly cleared by the liver. Dose adjustments may be required in hepatic impairment.
Paroxetine hydrochloride appears to be slowly but well absorbed from the GI tract following oral administration. Although the oral bioavailability of paroxetine hydrochloride in humans has not been fully elucidated to date, the manufacturer states that paroxetine is completely absorbed after oral dosing of a solution of the hydrochloride salt. However, the relative proportion of an oral dose that reaches systemic circulation unchanged appears to be relatively small because paroxetine undergoes extensive first-pass metabolism. The oral tablets and suspension of paroxetine hydrochloride reportedly are bioequivalent.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2359
In steady-state dose proportionality studies involving elderly and nonelderly patients, at doses of 20 mg to 40 mg daily for the elderly and 20 mg to 50 mg daily for the nonelderly, some nonlinearity was observed in both populations, again reflecting a saturable metabolic pathway. In comparison to Cmin values after 20 mg daily, values after 40 mg daily were only about 2 to 3 times greater than doubled.
NIH; DailyMed. Current Medication Information for Paxil (Paroxetine Hydrochloride Hemihydrate) Tablet, Film Coated; Paxil (Paroxetine Hydrochloride Hemihydrate) Suspension (Updated: July 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=584ace29-6e40-432f-950f-ab7e98653d32
Approximately 95% and 93% of paroxetine is bound to plasma protein at 100 ng/mL and 400 ng/mL, respectively. Under clinical conditions, paroxetine concentrations would normally be less than 400 ng/mL. Paroxetine does not alter the in vitro protein binding of phenytoin or warfarin.
NIH; DailyMed. Current Medication Information for Paxil (Paroxetine Hydrochloride Hemihydrate) Tablet, Film Coated; Paxil (Paroxetine Hydrochloride Hemihydrate) Suspension (Updated: July 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=584ace29-6e40-432f-950f-ab7e98653d32
Paroxetine distributes throughout the body, including the CNS, with only 1% remaining in the plasma.
NIH; DailyMed. Current Medication Information for Paxil (Paroxetine Hydrochloride Hemihydrate) Tablet, Film Coated; Paxil (Paroxetine Hydrochloride Hemihydrate) Suspension (Updated: July 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=584ace29-6e40-432f-950f-ab7e98653d32
For more Absorption, Distribution and Excretion (Complete) data for PAROXETINE (13 total), please visit the HSDB record page.
Paroxetine metabolism occurs in the liver and is largely mediated by cytochrome CYP2D6 with contributions from CYP3A4 and possibly other cytochrome enzymes. Genetic polymorphisms of the CYP2D6 enzyme may alter the pharmacokinetics of this drug. Poor metabolizers may demonstrate increased adverse effects while rapid metabolizers may experience decreased therapeutic effects. The majority of a paroxetine dose is oxidized to a catechol metabolite that is subsequently converted to both glucuronide and sulfate metabolites via methylation and conjugation. In rat synaptosomes, the glucuronide and sulfate conjugates have been shown to thousands of times less potent than paroxetine itself. The metabolites of paroxetine are considered inactive.
The exact metabolic fate of paroxetine has not been fully elucidated; however, paroxetine is extensively metabolized, probably in the liver. The principal metabolites are polar and conjugated products of oxidation and methylation, which are readily cleared by the body. Conjugates with glucuronic acid and sulfate predominate, and the principal metabolites have been isolated and identified. The metabolites of paroxetine have been shown to possess no more than 2% of the potency of the parent compound as inhibitors of serotonin reuptake; therefore, they are essentially inactive.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2359
Paroxetine is extensively metabolized after oral administration. The principal metabolites are polar and conjugated products of oxidation and methylation, which are readily cleared. Conjugates with glucuronic acid and sulfate predominate, and major metabolites have been isolated and identified. Data indicate that the metabolites have no more than 1/50 the potency of the parent compound at inhibiting serotonin uptake. The metabolism of paroxetine is accomplished in part by CYP2D6. Saturation of this enzyme at clinical doses appears to account for the nonlinearity of paroxetine kinetics with increasing dose and increasing duration of treatment. The role of this enzyme in paroxetine metabolism also suggests potential drug-drug interactions
NIH; DailyMed. Current Medication Information for Paxil (Paroxetine Hydrochloride Hemihydrate) Tablet, Film Coated; Paxil (Paroxetine Hydrochloride Hemihydrate) Suspension (Updated: July 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=584ace29-6e40-432f-950f-ab7e98653d32
Paroxetine has known human metabolites that include 4-[[(3S,4R)-4-(4-Fluorophenyl)piperidin-3-yl]methoxy]benzene-1,2-diol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The mean elimination half-life of paroxetine is about 21 hours. In healthy young subjects, mean elimination half-life was found to be 17.3 hours.
Paroxetine hydrochloride is completely absorbed after oral dosing of a solution of the hydrochloride salt. In a study in which normal male subjects (n = 15) received 30 mg tablets daily for 30 days, steady-state paroxetine concentrations were achieved by approximately 10 days for most subjects, although it may take substantially longer in an occasional patient. At steady state, mean ... half life was ... 21.0 hours (CV 32%) ... .
NIH; DailyMed. Current Medication Information for Paxil (Paroxetine Hydrochloride Hemihydrate) Tablet, Film Coated; Paxil (Paroxetine Hydrochloride Hemihydrate) Suspension (Updated: July 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=584ace29-6e40-432f-950f-ab7e98653d32
The mean elimination half-life is approximately 21 hours (CV 32%) after oral dosing of 30 mg tablets daily for 30 days of Paxil.
NIH; DailyMed. Current Medication Information for Paxil (Paroxetine Hydrochloride Hemihydrate) Tablet, Film Coated; Paxil (Paroxetine Hydrochloride Hemihydrate) Suspension (Updated: July 2014). Available from, as of May 1, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=584ace29-6e40-432f-950f-ab7e98653d32
The elimination half-life of paroxetine when administered as paroxetine hydrochloride averages approximately 21-24 hours, although there is wide interpatient variation with half-lives (ranging from 7-65 hours in one study). In healthy males receiving one 30-mg tablet of paroxetine (administered as paroxetine mesylate) once daily for 24 days, the mean paroxetine half-life was 33.2 hours. In geriatric individuals, elimination half-life of paroxetine (administered as paroxetine hydrochloride) may be increased (e.g., to about 36 hours).
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2359
Paroxetine enhances serotonergic activity via the inhibition presynaptic reuptake of serotonin by the serotonin (SERT) receptor. This inhibition raises the level of serotonin in the synaptic cleft, relieving various symptoms. This drug has been demonstrated to be a stronger inhibitor of serotonin reuptake than other members of the same drug class, including [Citalopram], [Fluoxetine], and [Fluvoxamine]. The mechanism of action of paroxetine in relieving the vasomotor symptoms of menopause is unknown, according to the Brisdelle prescribing information, but may occur due to its effects on thermoregulation. Paroxetine shows a clinically insignificant affinity for adrenergic alpha-1 and alpha-2 receptors and -adrenergic receptors, dopamine D1 and D2 receptors, histamine H1 receptors and serotonin 5-HT1A, 5-HT2A and 5-HT2C receptors. This drug shows some affinity for muscarinic cholinergic receptors and 5-H2B receptors. The delayed onset of paroxetine therapeutic effects may be explained by the initial paroxetine actions on the 5-HT neurons. In rats, paroxetine activates 5-HT1A receptors when it is first administered, inhibiting the stimulation of the 5-HT neurons and subsequent release of serotonin at the synaptic cleft.
Functional and structural approaches were used to examine the inhibitory mechanisms and binding site location for fluoxetine and paroxetine, two serotonin selective reuptake inhibitors, on nicotinic acetylcholine receptors (AChRs) in different conformational states. The results establish that: (a) fluoxetine and paroxetine inhibit h alpha1beta1 gammadelta AChR-induced Ca(2+) influx with higher potencies than dizocilpine. The potency of fluoxetine is increased approximately 10-fold after longer pre-incubation periods, which is in agreement with the enhancement of (3)H-cytisine binding to resting but activatable Torpedo AChRs elicited by these antidepressants, (b) fluoxetine and paroxetine inhibit the binding of the phencyclidine analog piperidyl-3,4-(3)H(N)]-(N-(1-(2 thienyl)cyclohexyl)-3,4-piperidine to the desensitized Torpedo AChR with higher affinities compared to the resting AChR, and (c) fluoxetine inhibits (3)H-dizocilpine binding to the desensitized AChR, suggesting a mutually exclusive interaction. This is supported by our molecular docking results where neutral dizocilpine and fluoxetine and the conformer of protonated fluoxetine with the highest LUDI score interact with the domain between the valine (position 13') and leucine (position 9') rings. Molecular mechanics calculations also evidence electrostatic interactions of protonated fluoxetine at positions 20', 21', and 24'. Protonated dizocilpine bridges these two binding domains by interacting with the valine and outer (position 20') rings. The high proportion of protonated fluoxetine and dizocilpine calculated at physiological pH suggests that the protonated drugs can be attracted to the channel mouth before binding deeper within the AChR ion channel between the leucine and valine rings, a domain shared with phencyclidine, finally blocking ion flux and inducing AChR desensitization.
PMID:20079457 Arias HR et al; Int J Biochem Cell Biol 42 (5): 712-24 (2010)
Paroxetine was shown to be a potent (Ki = 1.1 nM) and specific inhibitor of [3H]-5-hydroxytryptamine (5-HT) uptake into rat cortical and hypothalamic synaptosomes in vitro. Lineweaver-Burk kinetic analysis determined that this inhibition was competitive in nature, implying a direct interaction with the 5-HT uptake transporter complex. Oral administration of paroxetine produced a dose-related inhibition of [3H]-5-HT uptake (ED50 = 1.9 mg/kg) into rat hypothalamic synaptosomes ex vivo with little effect on [3H]-l-noradrenaline (NA) uptake (ED50 greater than 30 mg/kg). This selectivity for 5-HT uptake was maintained after oral dosing for 14 days. Paroxetine (ED50 1-3 mg/kg PO) prevented the 5-HT depleting effect of p-chloroamphetamine (PCA) in rat brain, demonstrating 5-HT uptake blockade in vivo. Radioligand binding techniques in rat brain in vitro showed that paroxetine has little affinity for alpha 1, alpha 2 or beta adrenoceptors, dopamine (D2), 5-HT1, 5-HT2 or histamine (H1) receptors at concentrations below 1000 nM. Paroxetine demonstrated weak affinity for muscarinic receptors (Ki = 89 nM) but was at least 15 fold weaker than amitriptyline (Ki = 5.1 nM). Paroxetine, therefore, provides a useful pharmacological tool for investigating 5-HT systems and furthermore should be an antidepressant with reduced tricyclic-like side-effects.
PMID:2962217 Thomas DR et al; Psychopharmacology (Berl) 93 (2): 193-200 (1987)
The precise mechanism of antidepressant action of paroxetine is unclear, but the drug has been shown to selectively inhibit the reuptake of serotonin at the presynaptic neuronal membrane. Paroxetine-induced inhibition of serotonin reuptake causes increased synaptic concentrations of serotonin in the CNS, resulting in numerous functional changes associated with enhanced serotonergic neurotransmission. Like other SSRIs (e.g., citalopram, fluoxetine, fluvoxamine, sertraline), paroxetine appears to have only very weak effects on the reuptake of norepinephrine or dopamine and does not exhibit clinically important anticholinergic, antihistaminic, or adrenergic (a1, a2, beta) blocking activity at usual therapeutic dosages. Although the mechanism of antidepressant action of antidepressant agents may involve inhibition of the reuptake of various neurotransmitters (i.e., serotonin, norepinephrine) at the presynaptic neuronal membrane, it has been suggested that postsynaptic receptor modification is mainly responsible for the antidepressant action observed during long-term administration of antidepressant agents. During long-term therapy with most antidepressants (e.g., tricyclic antidepressants, monoamine oxidase (MAO) inhibitors), these adaptive changes mainly consist of subsensitivity of the noradrenergic adenylate cyclase system in association with a decrease in the number of beta-adrenergic receptors; such effects on noradrenergic receptor function are commonly referred to as down regulation. However, in an animal study, long-term administration of paroxetine was not shown to downregulate noradrenergic receptors in the CNS as has been observed with many other clinically effective antidepressants. In addition, some antidepressants (e.g., amitriptyline) reportedly decrease the number of serotonergic (5-HT) binding sites following chronic administration.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2357
Reduced glucose metabolism has been implicated as a pathophysiology of depressive disorder. Normalization of such impaired neurometabolism has been related to the therapeutic actions of antidepressant medication. However, the molecular mechanism underlying the neurometabolic actions of antidepressants has not been fully understood. Given that AMP-activated protein kinase (AMPK) is a master switch for energy homeostasis, we aimed to determine whether selective serotonin reuptake inhibitor paroxetine enhances energy metabolism by activating AMPK in neuroblastoma cells. We found that paroxetine dose dependently increased mitochondrial biogenesis, which involves the AMPK-peroxisome proliferator-activated receptor-gamma coactivator-1a pathway. In addition, paroxetine-induced AMPK activation increases glucose uptake and ATP production. These neurometabolic effects of paroxetine were suppressed by cotreatment with compound C (CC), an AMPK inhibitor. These findings suggest a possibility that modulation of the AMPK pathway might be a previously unrecognized mechanism underlying the neurometabolic action of antidepressants. Further study is warranted to examine the region-specific and time-specific effects of AMPK modulation by antidepressants on mood-related behaviors.
PMID:25839176 Jeong J et al; Neuroreport 26 (7): 424-8 (2015)
For more Mechanism of Action (Complete) data for PAROXETINE (9 total), please visit the HSDB record page.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
73
PharmaCompass offers a list of Paroxetine Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Paroxetine Hydrochloride manufacturer or Paroxetine Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Paroxetine Hydrochloride manufacturer or Paroxetine Hydrochloride supplier.
PharmaCompass also assists you with knowing the Paroxetine Hydrochloride API Price utilized in the formulation of products. Paroxetine Hydrochloride API Price is not always fixed or binding as the Paroxetine Hydrochloride Price is obtained through a variety of data sources. The Paroxetine Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Paroxetine Hydrochloride Hemihydrate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Paroxetine Hydrochloride Hemihydrate, including repackagers and relabelers. The FDA regulates Paroxetine Hydrochloride Hemihydrate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Paroxetine Hydrochloride Hemihydrate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Paroxetine Hydrochloride Hemihydrate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Paroxetine Hydrochloride Hemihydrate supplier is an individual or a company that provides Paroxetine Hydrochloride Hemihydrate active pharmaceutical ingredient (API) or Paroxetine Hydrochloride Hemihydrate finished formulations upon request. The Paroxetine Hydrochloride Hemihydrate suppliers may include Paroxetine Hydrochloride Hemihydrate API manufacturers, exporters, distributors and traders.
click here to find a list of Paroxetine Hydrochloride Hemihydrate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Paroxetine Hydrochloride Hemihydrate DMF (Drug Master File) is a document detailing the whole manufacturing process of Paroxetine Hydrochloride Hemihydrate active pharmaceutical ingredient (API) in detail. Different forms of Paroxetine Hydrochloride Hemihydrate DMFs exist exist since differing nations have different regulations, such as Paroxetine Hydrochloride Hemihydrate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Paroxetine Hydrochloride Hemihydrate DMF submitted to regulatory agencies in the US is known as a USDMF. Paroxetine Hydrochloride Hemihydrate USDMF includes data on Paroxetine Hydrochloride Hemihydrate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Paroxetine Hydrochloride Hemihydrate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Paroxetine Hydrochloride Hemihydrate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Paroxetine Hydrochloride Hemihydrate Drug Master File in Japan (Paroxetine Hydrochloride Hemihydrate JDMF) empowers Paroxetine Hydrochloride Hemihydrate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Paroxetine Hydrochloride Hemihydrate JDMF during the approval evaluation for pharmaceutical products. At the time of Paroxetine Hydrochloride Hemihydrate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Paroxetine Hydrochloride Hemihydrate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Paroxetine Hydrochloride Hemihydrate Drug Master File in Korea (Paroxetine Hydrochloride Hemihydrate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Paroxetine Hydrochloride Hemihydrate. The MFDS reviews the Paroxetine Hydrochloride Hemihydrate KDMF as part of the drug registration process and uses the information provided in the Paroxetine Hydrochloride Hemihydrate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Paroxetine Hydrochloride Hemihydrate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Paroxetine Hydrochloride Hemihydrate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Paroxetine Hydrochloride Hemihydrate suppliers with KDMF on PharmaCompass.
A Paroxetine Hydrochloride Hemihydrate CEP of the European Pharmacopoeia monograph is often referred to as a Paroxetine Hydrochloride Hemihydrate Certificate of Suitability (COS). The purpose of a Paroxetine Hydrochloride Hemihydrate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Paroxetine Hydrochloride Hemihydrate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Paroxetine Hydrochloride Hemihydrate to their clients by showing that a Paroxetine Hydrochloride Hemihydrate CEP has been issued for it. The manufacturer submits a Paroxetine Hydrochloride Hemihydrate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Paroxetine Hydrochloride Hemihydrate CEP holder for the record. Additionally, the data presented in the Paroxetine Hydrochloride Hemihydrate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Paroxetine Hydrochloride Hemihydrate DMF.
A Paroxetine Hydrochloride Hemihydrate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Paroxetine Hydrochloride Hemihydrate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Paroxetine Hydrochloride Hemihydrate suppliers with CEP (COS) on PharmaCompass.
A Paroxetine Hydrochloride Hemihydrate written confirmation (Paroxetine Hydrochloride Hemihydrate WC) is an official document issued by a regulatory agency to a Paroxetine Hydrochloride Hemihydrate manufacturer, verifying that the manufacturing facility of a Paroxetine Hydrochloride Hemihydrate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Paroxetine Hydrochloride Hemihydrate APIs or Paroxetine Hydrochloride Hemihydrate finished pharmaceutical products to another nation, regulatory agencies frequently require a Paroxetine Hydrochloride Hemihydrate WC (written confirmation) as part of the regulatory process.
click here to find a list of Paroxetine Hydrochloride Hemihydrate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Paroxetine Hydrochloride Hemihydrate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Paroxetine Hydrochloride Hemihydrate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Paroxetine Hydrochloride Hemihydrate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Paroxetine Hydrochloride Hemihydrate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Paroxetine Hydrochloride Hemihydrate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Paroxetine Hydrochloride Hemihydrate suppliers with NDC on PharmaCompass.
Paroxetine Hydrochloride Hemihydrate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Paroxetine Hydrochloride Hemihydrate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Paroxetine Hydrochloride Hemihydrate GMP manufacturer or Paroxetine Hydrochloride Hemihydrate GMP API supplier for your needs.
A Paroxetine Hydrochloride Hemihydrate CoA (Certificate of Analysis) is a formal document that attests to Paroxetine Hydrochloride Hemihydrate's compliance with Paroxetine Hydrochloride Hemihydrate specifications and serves as a tool for batch-level quality control.
Paroxetine Hydrochloride Hemihydrate CoA mostly includes findings from lab analyses of a specific batch. For each Paroxetine Hydrochloride Hemihydrate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Paroxetine Hydrochloride Hemihydrate may be tested according to a variety of international standards, such as European Pharmacopoeia (Paroxetine Hydrochloride Hemihydrate EP), Paroxetine Hydrochloride Hemihydrate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Paroxetine Hydrochloride Hemihydrate USP).

