



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
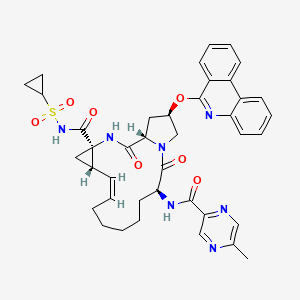

1. (2r,6s,12z,13as,14ar,16as)-n-(cyclopropylsulfonyl)-6-(5-methylpyrazin-2-carboxamido)-5,16-dioxo-2-(phenanthridin-6-yloxy)-1,2,3,6,7,8,9,10,11,13a,14,15,16,16atetradecahydrocyclopropa(e)pyrrolo(1,2-a)(1,4)diazacyclopentadecine-14a(5h)-carboxamide
2. Abt 450
3. Abt-450
4. Abt450
5. Paritaprevir Dihydrate
6. Paritaprevir Hydrate
7. Veruprevir
8. Veruprevir Anhydrous
1. Veruprevir
2. Abt-450
3. Veruprevir Anhydrous
4. 1216941-48-8
5. Abt450
6. 1221573-85-8
7. Ou2ym37k86
8. (2r,6s,12z,13as,14ar,16as)-n-(cyclopropylsulfonyl)-6-(5-methylpyrazin-2-carboxamido)-5,16-dioxo-2-(phenanthridin-6-yloxy)-1,2,3,6,7,8,9,10,11,13a,14,15,16,16atetradecahydrocyclopropa(e)pyrrolo(1,2-a)(1,4)diazacyclopentadecine-14a(5h)-carboxamide
9. Paritaprevir(abt-450)
10. (2r,6s,13as,14ar,16as,z)-n-(cyclopropylsulfonyl)-6-(5-methylpyrazine-2-carboxamido)-5,16-dioxo-2-(phenanthridin-6-yloxy)-1,2,3,5,6,7,8,9,10,11,13a,14,14a,15,16,16a-hexadecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a-carboxamide
11. Abt 450
12. Veruprevir [inn]
13. Paritaprevir [usan]
14. Paritaprevir [usan:inn]
15. Unii-ou2ym37k86
16. Paritaprevir [mi]
17. Paritaprevir [inn]
18. Paritaprevir [vandf]
19. Veruprevir (deprecated Inn)
20. Paritaprevir [who-dd]
21. Schembl3069964
22. Chembl3391662
23. Gtpl11273
24. Amy6938
25. Dtxsid601027922
26. Paritaprevir [orange Book]
27. Paritaprevir(veruprevir Abt-450)
28. (2r,6s,12z,13ar,14ar,16as)-n-(cyclopropanesulfonyl)-6-[(5-methylpyrazine-2-carbonyl)amino]-5,16-dioxo-2-[(phenanthridin-6-yl)oxy]-1,2,3,6,7,8,9,10,11,13a,14,15,16,16a-tetradecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a(5h)-carboxamide
29. Ex-a2278
30. S5404
31. Viekirax Component Paritaprevir
32. Akos025396424
33. Zinc197964623
34. Ccg-270449
35. Cs-5051
36. Db09297
37. Ncgc00509859-02
38. Paritaprevir Component Of Viekirax
39. Ac-33061
40. As-75348
41. Hy-12594
42. A857160
43. (1s,4r,6s,7z,14s,18r)-n-(cyclopropanesulfonyl)-14-(5-methylpyrazine-2-amido)-2,15-dioxo-18-(phenanthridin-6-yloxy)-3,16-diazatricyclo[14.3.0.0?,?]nonadec-7-ene-4-carboxamide
44. (1s,4r,6s,7z,14s,18r)-n-cyclopropylsulfonyl-14-[(5-methylpyrazine-2-carbonyl)amino]-2,15-dioxo-18-phenanthridin-6-yloxy-3,16-diazatricyclo[14.3.0.04,6]nonadec-7-ene-4-carboxamide
45. (2r,6s,12z,13as,14ar,16as)-n-(cyclopropylsulfonyl)-6-{[(5-methyl-2-pyrazinyl)carbonyl]amino}-5,16-dioxo-2-(6-phenanthridinyloxy)-1,2,3,6,7,8,9,10,11,13a,14,15,16,16a-tetradecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a(5h)-carboxamide
46. (2r,6s,13as,14ar,16as,z)-n-(cyclopropylsulfonyl)-6-(5-methylpyrazine-2-carboxamido)-5,16-dioxo-2-(phenanthridin-6-yloxy)-1,2,3,6,7,8,9,10,11,13a,14,15,16,16a-tetradecahydrocyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a(5h)-carboxamide
47. Cyclopropa[e]pyrrolo[1,2-a][1,4]diazacyclopentadecine-14a(5h)-carboxamide, N-(cyclopropylsulfonyl)-1,2,3,6,7,8,9,10,11,13a,14,15,16,16a-tetradecahydro-6-[[(5-methyl-2-pyrazinyl)carbonyl]amino]-5,16-dioxo-2-(6-phenanthridinyloxy)-, (2r,6s,12z,13as,14ar,16as)-
| Molecular Weight | 765.9 g/mol |
|---|---|
| Molecular Formula | C40H43N7O7S |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 765.29446791 g/mol |
| Monoisotopic Mass | 765.29446791 g/mol |
| Topological Polar Surface Area | 198 Ų |
| Heavy Atom Count | 55 |
| Formal Charge | 0 |
| Complexity | 1600 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
When used within the fixed-dose combination product with [DB09296], [DB09183], and [DB00503] as the FDA-approved product Viekira Pak, paritaprevir is indicated for the treatment of HCV genotype 1b without cirrhosis or with compensated cirrhosis, and when combined with [DB00811] for the treatment of HCV genotype 1a without cirrhosis or with compensated cirrhosis. When used within the fixed-dose combination product with [DB09296] and [DB00503] as the FDA- and Health Canada-approved product Technivie, paritaprevir is indicated in combination with [DB00811] for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis. When used within the fixed-dose combination product with [DB09296], [DB09183], and [DB00503] as the Health Canada-approved, commercially available product Holkira Pak, paritaprevir is indicated for the treatment of HCV genotype 1b with or without cirrhosis, and when combined with [DB00811] for the treatment of HCV genotype 1a with or without cirrhosis.
FDA Label
At concentrations approximately 6 and 1.8 times the therapeutic concentrations of paritaprevir and ombitasvir, the combination did not prolong QTc to any clinically relevant extent.
Absorption
Tmax of approximately 4 to 5 hours with a maximum concentration (Cmax) of 194 ng/mL.
Route of Elimination
Following a single dose administration of 14C-paritaprevir co-dosed with 100 mg of ritonavir, approximately 88% of the radioactivity was recovered in feces with limited radioactivity (8.8%) in urine; unchanged paritaprevir accounted for 1.1% of the radioactivity in the feces and 0.05% in the urine.
Volume of Distribution
Volume of distribution at steady state is approximately 103 L.
Paritaprevir is predominantly metabolized by CYP3A4 and to a lesser extent by CYP3A5.
5.5 hr
Paritaprevir is a potent inhibitor of the NS3/4A serine protease of Hepatitis C Virus (HCV). Following viral replication of HCV genetic material and translation into a single polypeptide, Nonstructural Protein 3 (NS3) and its activating cofactor Nonstructural Protein 4A (NS4A) are responsible for cleaving it into the following structural and nonstructural proteins required for assembly into mature virus: NS3, NS4A, NS4B, NS5A, and NS5B. By inhibiting viral protease NS3/4A, paritaprevir therefore prevents viral replication and function.




