
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada
0
Australia
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
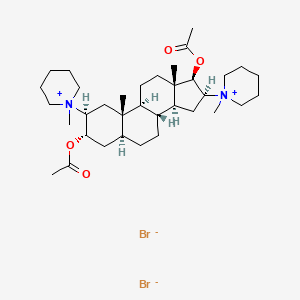

1. Bromide, Pancuronium
2. Pancuronium
3. Pancuronium Curamed
4. Pancuronium Organon
5. Pavulon
1. Pancuronium Dibromide
2. 15500-66-0
3. Pavulon
4. Mioblock
5. Org Na 97
6. Pancuronium (dibromide)
7. Pancuronii Bromidum
8. Bromuro De Pancuronio
9. Bromure De Pancuronium
10. Bromurex
11. Org-na 97
12. Chebi:7908
13. Org-na-97
14. 3alpha,17beta-diacetoxy-2beta,16beta-dipiperidino-5alpha-androstane Dimethobromide
15. Na 97
16. 2beta,16beta-dipiperidino-5alpha-androstane-3alpha,17beta-diol Diacetate Dimethobromide
17. U9ly9y75x2
18. Bromure De Pancuronium [inn-french]
19. Bromuro De Pancuronio [inn-spanish]
20. 15500-66-0 (bromide)
21. Nsc-293162
22. 1,1'-((2s,3s,5s,8r,9s,10s,13s,14s,16s,17r)-3,17-diacetoxy-10,13-dimethylhexadecahydro-1h-cyclopenta[a]phenanthrene-2,16-diyl)bis(1-methylpiperidin-1-ium) Bromide
23. (2beta,3alpha,5alpha,16beta,17beta)-3,17-bis(acetyloxy)-2,16-bis(1-methylpiperidinium-1-yl)androstane Dibromide
24. 3alpha,17beta-diacetoxy-2beta,16beta-bis(1-methylpiperidinium-1-yl)-5alpha-androstane Dibromide
25. Hsdb 3244
26. Pancuronii Bromidum [inn-latin]
27. Einecs 239-532-5
28. Nsc 293162
29. Unii-u9ly9y75x2
30. Pavulon (tn)
31. Mfcd00079223
32. Ncgc00163232-01
33. Dsstox_cid_3415
34. Pancuronium Bromide [usan:usp:inn:ban:jan]
35. Cas-15500-66-0
36. 1,1'-(3,17-bis(acetyloxy)androstane-2,16-diyl)bis(1-methylpiperidinium) Dibromide
37. 2beta,16beta-dipiperidino-5alpha-androstane-3alpha,17beta-dioldiacetatedimethobromide
38. 3-alpha,17-beta-diacetoxy-2-beta,16-beta-dipiperidino-5-alpha-androstane Dimethobromide
39. 5alpha-androstan-3alpha,17beta-diol, 2beta,16beta-dipipecolinio-, Dibromide, Diacetate
40. Dsstox_rid_77018
41. Dsstox_gsid_23415
42. Schembl41185
43. 1,1'-(3alpha,17beta-bis(acetyloxy)-5alpha-androstane-2beta,16beta-diyl)bis(1-methylpiperidinium) Dibromide
44. 1,1'-(3alpha,17beta-dihydroxy-5alpha-androstan-2beta,16beta-ylene)bis(1-methylpiperidinium) Dibromide Diacetate
45. Piperidinium, 1,1'-((2beta,3alpha,5alpha,16beta,17beta)-3,17-bis(acetyloxy)androstane-2,16-diyl)bis(1-methyl-, Dibromide
46. Piperidinium, 1,1'-(2-beta,16-beta-(3-alpha,17-beta-dihydroxy-5-alpha-androstanylene))bis(1-methyl-, Dibromide, Diacetate
47. Piperidinium, 1,1'-(3alpha,17beta-dihydroxy-5alpha-androstan-2beta,16beta-ylene)bis(1-methyl-, Dibromide, Diacetate
48. Chembl1200757
49. Pancuronium Bromide [mi]
50. Pancuronium Bromide [inn]
51. Pancuronium Bromide [jan]
52. Hms1571o09
53. Hms2098o09
54. Hms3262b16
55. Hms3715o09
56. Hms3884p21
57. Pancuronium Bromide [hsdb]
58. Pancuronium Bromide [usan]
59. Pancuronium Bromide [vandf]
60. Hy-b0429
61. Pancuronium Bromide [mart.]
62. Tox21_112033
63. Tox21_500887
64. Bdbm50248016
65. Pancuronium Bromide [usp-rs]
66. Pancuronium Bromide [who-dd]
67. S2497
68. Akos037515715
69. Pancuronium Bromide (jp17/usp/inn)
70. Ccg-221034
71. Ccg-222191
72. Lp00887
73. Ncgc00261572-01
74. Pancuronium Bromide [orange Book]
75. Bs-15969
76. Pancuronium Bromide [ep Monograph]
77. Piperidinium, 1,1'-((2beta,3alpha,5alpha,16beta,17beta)-3,17-bis(acetyloxy)androstane-2,16-diyl)bis(1-methyl)-, Dibromide
78. Pancuronium Bromide [usp Monograph]
79. Eu-0100887
80. D00492
81. P 1918
82. A809594
83. Sr-01000000127
84. Sr-01000076059
85. Q-101015
86. Sr-01000000127-3
87. Sr-01000076059-1
88. Q27107612
89. Pancuronium Bromide, European Pharmacopoeia (ep) Reference Standard
90. Pancuronium Bromide, United States Pharmacopeia (usp) Reference Standard
91. Vecuronium Bromide Impurity, Pancuronium Bromide- [usp Impurity]
92. Pancuronium Bromide For System Suitability, European Pharmacopoeia (ep) Reference Standard
93. [(2s,3s,5s,8r,9s,10s,13s,14s,16s,17r)-17-acetyloxy-10,13-dimethyl-2,16-bis(1-methylpiperidin-1-ium-1-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl] Acetate;bromide
94. [(2s,3s,5s,8r,9s,10s,13s,14s,16s,17r)-17-acetyloxy-10,13-dimethyl-2,16-bis(1-methylpiperidin-1-ium-1-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl] Acetate;dibromide
95. [17-acetoxy-10,13-dimethyl-2,16-bis(1-methylpiperidin-1-ium-1-yl)-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-3-yl] Acetate Dibromide
96. 1,1'-((2s,3s,5s,8r,9s,10s,13s,14s,16s,17r)-3,17-diacetoxy-10,13-dimethylhexadecahydro-1h-cyclopenta[a]phenanthrene-2,16-diyl)bis(1-methylpiperidin-1-ium) Bromi
97. 1,1'-([2beta,3alpha,5alpha,16beta,17beta]-3,17-bis[acetyloxy]androstane-2,16-diyl)bis(1-methylpiperidinium) Dibromide
98. 1,1'-(3.alpha.,17.beta.-dihydroxy-5.alpha.-androstan-2.beta.,16.beta.-ylene)bis(1-methylpiperidinium)dibromide Diacetate
99. 1,1'-(3alpha,17beta-dihydroxy-2beta,5alpha-androstan-2beta,16beta-ylene) Bis[1-methylpiperidinium] Diacetate Dibromide
100. 2.beta.,16.beta.-dipiperidino-5.alpha.-androstane-3.alpha.,17.beta.-diol Diacetate Dimethobromide
101. Piperidinium, 1,1'-((2.beta.,3.alpha.,5.alpha.,16.beta.,17.beta.)-3,17-bis(acetyloxy)androstane-2,16-diyl)bis(1-methyl)-, Dibromide
| Molecular Weight | 732.7 g/mol |
|---|---|
| Molecular Formula | C35H60Br2N2O4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 732.28993 g/mol |
| Monoisotopic Mass | 730.29198 g/mol |
| Topological Polar Surface Area | 52.6 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 1000 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Pancuronium bromide |
| Drug Label | Pancuronium Bromide is a nondepolarizing neuromuscular blocking agent chemically designated as the aminosteroid 2, 16 - dipiperidino-5-androstane-3, 17- diol diacetate dimethobromide, C35H60Br2N2O4. It is a fine white odorless powder which... |
| Active Ingredient | Pancuronium bromide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml; 1mg/ml |
| Market Status | Prescription |
| Company | Hospira; Teva Pharms Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Pancuronium bromide |
| Drug Label | Pancuronium Bromide is a nondepolarizing neuromuscular blocking agent chemically designated as the aminosteroid 2, 16 - dipiperidino-5-androstane-3, 17- diol diacetate dimethobromide, C35H60Br2N2O4. It is a fine white odorless powder which... |
| Active Ingredient | Pancuronium bromide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2mg/ml; 1mg/ml |
| Market Status | Prescription |
| Company | Hospira; Teva Pharms Usa |
Neuromuscular Nondepolarizing Agents; Nicotinic Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
THE MAIN CLINICAL USE OF THE NEUROMUSCULAR BLOCKING AGENTS IS AS AN ADJUVANT IN SURGICAL ANESTHESIA TO OBTAIN RELAXATION OF SKELETAL MUSCLE, PARTICULARLY OF THE ABDOMINAL WALL ... MUSCLE RELAXATION IS ALSO OF VALUE IN VARIOUS ORTHOPEDIC PROCEDURES, SUCH AS THE CORRECTION OF DISLOCATIONS & THE ALIGNMENT OF FRACTURES. /NEUROMUSCULAR BLOCKING AGENTS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 189
...MAY BE USED MORE SAFELY IN PT WITH CARDIOVASCULAR DISEASE OR BRONCHIAL ASTHMA THAN ANY OTHER NEUROMUSCULAR BLOCKING DRUG. ...IT HAS ACTUALLY BEEN USED IN MGMNT OF STATUS ASTHMATICUS TO RELAX MUSCLES, THEREBY FACILITATING ARTIFICIAL RESPIRATION & DECR OXYGEN DEMAND. ... DURATION OF ACTION OF USUAL DOSE IS GENERALLY 30-60 MIN...
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 864
/NEUROMUSCULAR BLOCKING AGENTS/ HAVE BEEN USED TO FACILITATE LARYNGOSCOPY, BRONCHOSCOPY, & ESOPHAGOSCOPY, IN COMBINATION WITH A GENERAL ANESTHETIC AGENT. /NEUROMUSCULAR BLOCKING AGENTS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 190
For more Therapeutic Uses (Complete) data for PANCURONIUM BROMIDE (9 total), please visit the HSDB record page.
THE NEUROMUSCULAR BLOCKING AGENTS ARE POTENTIALLY HAZARDOUS DRUGS. CONSEQUENTLY, THEY SHOULD BE ADMINISTERED TO PATIENTS ONLY BY ANESTHESIOLOGISTS & OTHER CLINICIANS WHO HAVE HAD EXTENSIVE TRAINING IN THEIR USE & IN A SETTING WHERE FACILITIES FOR RESPIRATORY & CARDIOVASCULAR RESUSCITATION ARE IMMEDIATELY AT HAND. /NEUROMUSCULAR BLOCKING AGENTS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 190
...IT IS ADVISABLE TO USE DRUG CAUTIOUSLY IN PRESENCE OF RENAL OR LIVER DISEASES.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 864
EFFECT OF SPECIFIC DOSE OF ... PANCURONIUM MAY /POSSIBLY/ BE REDUCED IN PT WITH HIGH PLASMA GLOBULIN LEVELS (EG THOSE WITH LIVER DISEASE).
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 310
GREAT CARE SHOULD BE TAKEN WHEN ADMIN MUSCLE RELAXANTS TO DEHYDRATED OR SEVERELY ILL PATIENTS. /NEUROMUSCULAR BLOCKING AGENTS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 188
For more Drug Warnings (Complete) data for PANCURONIUM BROMIDE (17 total), please visit the HSDB record page.
Neuromuscular Nondepolarizing Agents
Drugs that interrupt transmission at the skeletal neuromuscular junction without causing depolarization of the motor end plate. They prevent acetylcholine from triggering muscle contraction and are used as muscle relaxants during electroshock treatments, in convulsive states, and as anesthesia adjuvants. (See all compounds classified as Neuromuscular Nondepolarizing Agents.)
Nicotinic Antagonists
Drugs that bind to nicotinic cholinergic receptors (RECEPTORS, NICOTINIC) and block the actions of acetylcholine or cholinergic agonists. Nicotinic antagonists block synaptic transmission at autonomic ganglia, the skeletal neuromuscular junction, and at central nervous system nicotinic synapses. (See all compounds classified as Nicotinic Antagonists.)
BOTH LIVER & KIDNEYS ARE INVOLVED IN DEGRADATION & EXCRETION OF ... PANCURONIUM ...
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 310
AFTER IV INJECTION, EFFECTS...BECOME MAXIMAL IN LESS THAN 3 MIN IN ADULTS & 90 SEC IN CHILDREN. ... PLASMA HALF-LIFE IS PROBABLY SLIGHTLY LESS THAN 2 HR. PANCURONIUM IS MOSTLY EXCRETED UNCHANGED INTO URINE.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 864
PLACENTAL TRANSFER OF...PANCURONIUM BROMIDE...OCCURS RAPIDLY AFTER ADMIN TO MOTHERS, BUT FETAL:MATERNAL DRUG CONCN RATIO ARE VERY LOW.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 156
PLASMA LEVELS OF PANCURONIUM OBEYED TWO-COMPARTMENT KINETICS IN SEVEN PATIENTS ON IV INJECTION & THE BETA-PHASE HALF-TIME VARIED BETWEEN 90 AND 162 MIN. THE MEAN VOLUME OF THE CENTRAL COMPARTMENT WAS 100 ML/KG, WHILE THE OVERALL DISTRIBUTION VOLUME WAS 261 MG/KG. IN PATIENTS WITH CHRONIC RENAL FAILURE, THE PLASMA CLEARANCE...WAS SIGNIFICANTLY REDUCED, WHILE VOLUMES OF BOTH THE OVERALL & CENTRAL COMPARTMENTS WERE SIGNIFICANTLY INCREASED.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 5: A Review of the Literature Published during 1976 and 1977. London: The Chemical Society, 1979., p. 57
For more Absorption, Distribution and Excretion (Complete) data for PANCURONIUM BROMIDE (6 total), please visit the HSDB record page.
IN CATS, 8 HR AFTER IV INJECTION OF PANCURONIUM BROMIDE, UNCHANGED PANCURONIUM BROMIDE IN URINE, BILE, & LIVER ACCOUNTED FOR 58% OF DOSE, 3-HYDROXY-DERIV FOR 14.5%, 17-HYDROXY-DERIV FOR 7% & 3,17-DIHYDROXY-DERIV FOR 4.5%.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 273
PLASMA HALF-LIFE IS PROBABLY SLIGHTLY LESS THAN 2 HR.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 864
LOW CONCN OF PANCURONIUM BROMIDE (5X10-8 G/ML OR LESS), HAD NO PRESYNAPTIC EFFECT ON MURINE PHRENIC NERVE-DIAPHRAGM PREPN. AT HIGH CONCN (5X10-7 G/ML), PANCURONIUM BROMIDE DEPRESSED QUANTAL RELEASE TO 26% OF CONTROL IN CUT-FIBER PREPN & 40% OF CONTROL IN HIGH-MAGNESIUM PREPN. POSTSYNAPTIC EFFECTS REVEALED DEPRESSION TO 16 & 22% OF CONTROL, RESPECTIVELY, AT A CONCN OF 5X10-7 G/ML. PANCURONIUM BROMIDE HAD NO EFFECT ON DIRECTLY ELICITED ACTION POTENTIALS & ELECTRIC MEMBRANE CONSTANTS. THUS, PRESYNAPTIC AS WELL AS POSTSYNAPTIC EFFECTS OF PANCURONIUM BROMIDE IN PARALYTIC DOSES ARE ESSENTIAL IN CONTRIBUTING TO THE TOTAL EFFICACY OF NEUROMUSCULAR DEPRESSION.
SU PC ET AL; PRE- AND POSTSYNAPTIC EFFECTS OF PANCURONIUM AT THE NEUROMUSCULAR JUNCTION OF THE MOUSE; ANESTHESIOLOGY 50(3) 199 (1979)
THE PHARMACODYNAMICS OF D-TUBOCURARINE (D-TC), PANCURONIUM BROMIDE, METOCURINE, & GALLAMINE WERE STUDIED IN RAT PHRENIC NERVE-HEMIDIAPHRAGM PREPN WITH VASCULAR PERFUSION AT 25, 31, & 37 C. D-TC, METOCURINE, & GALLAMINE EACH DEMONSTRATED A NEAR 2-FOLD INCREASE IN ED50 AT 25 C COMPARED WITH 37 C. NO SUCH RELATIONSHIP WAS APPARENT WITH PANCURONIUM BROMIDE. SLOPES OF THE DOSE-RESPONSE CURVES WERE NOT INFLUENCED BY TEMP; HOWEVER, THE SLOPES FOR METOCURINE & D-TC WERE LOWER THAN THOSE FOR PANCURONIUM BROMIDE & GALLAMINE. THUS, IN THE RAT, PANCURONIUM BROMIDE RETAINS POTENCY AT HYPOTHERMIA, WHEREAS THE OTHER RELAXANTS DECREASE POTENCY. IN ADDITION, METOCURINE & D-TC EXHIBIT LESS STEEP DOSE-RESPONSE CURVES UNDER THESE EXPTL CONDITIONS.
HORROW JC, BARTKOWSKI RR; PANCURONIUM, UNLIKE OTHER NONDEPOLARIZING RELAXANTS, RETAINS POTENCY AT HYPOTHERMIA; ANESTHESIOLOGY 58(4) 357 (1983)
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
19
PharmaCompass offers a list of Pancuronium Bromide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Pancuronium Bromide manufacturer or Pancuronium Bromide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Pancuronium Bromide manufacturer or Pancuronium Bromide supplier.
PharmaCompass also assists you with knowing the Pancuronium Bromide API Price utilized in the formulation of products. Pancuronium Bromide API Price is not always fixed or binding as the Pancuronium Bromide Price is obtained through a variety of data sources. The Pancuronium Bromide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Pancuronium Bromide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Pancuronium Bromide, including repackagers and relabelers. The FDA regulates Pancuronium Bromide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Pancuronium Bromide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Pancuronium Bromide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Pancuronium Bromide supplier is an individual or a company that provides Pancuronium Bromide active pharmaceutical ingredient (API) or Pancuronium Bromide finished formulations upon request. The Pancuronium Bromide suppliers may include Pancuronium Bromide API manufacturers, exporters, distributors and traders.
click here to find a list of Pancuronium Bromide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Pancuronium Bromide DMF (Drug Master File) is a document detailing the whole manufacturing process of Pancuronium Bromide active pharmaceutical ingredient (API) in detail. Different forms of Pancuronium Bromide DMFs exist exist since differing nations have different regulations, such as Pancuronium Bromide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Pancuronium Bromide DMF submitted to regulatory agencies in the US is known as a USDMF. Pancuronium Bromide USDMF includes data on Pancuronium Bromide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Pancuronium Bromide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Pancuronium Bromide suppliers with USDMF on PharmaCompass.
A Pancuronium Bromide CEP of the European Pharmacopoeia monograph is often referred to as a Pancuronium Bromide Certificate of Suitability (COS). The purpose of a Pancuronium Bromide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Pancuronium Bromide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Pancuronium Bromide to their clients by showing that a Pancuronium Bromide CEP has been issued for it. The manufacturer submits a Pancuronium Bromide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Pancuronium Bromide CEP holder for the record. Additionally, the data presented in the Pancuronium Bromide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Pancuronium Bromide DMF.
A Pancuronium Bromide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Pancuronium Bromide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Pancuronium Bromide suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Pancuronium Bromide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Pancuronium Bromide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Pancuronium Bromide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Pancuronium Bromide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Pancuronium Bromide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Pancuronium Bromide suppliers with NDC on PharmaCompass.
Pancuronium Bromide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Pancuronium Bromide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Pancuronium Bromide GMP manufacturer or Pancuronium Bromide GMP API supplier for your needs.
A Pancuronium Bromide CoA (Certificate of Analysis) is a formal document that attests to Pancuronium Bromide's compliance with Pancuronium Bromide specifications and serves as a tool for batch-level quality control.
Pancuronium Bromide CoA mostly includes findings from lab analyses of a specific batch. For each Pancuronium Bromide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Pancuronium Bromide may be tested according to a variety of international standards, such as European Pharmacopoeia (Pancuronium Bromide EP), Pancuronium Bromide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Pancuronium Bromide USP).

