



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
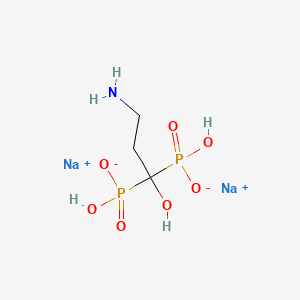

1. (3-amino-1-hydroxypropylidene)-1,1-biphosphonate
2. 1 Hydroxy 3 Aminopropane 1,1 Diphosphonic Acid
3. 1-hydroxy-3-aminopropane-1,1-diphosphonic Acid
4. Ahprbp
5. Amidronate
6. Amino 1 Hydroxypropane 1,1 Diphosphonate
7. Amino-1-hydroxypropane-1,1-diphosphonate
8. Aminohydroxypropylidene Diphosphonate
9. Aminopropanehydroxydiphosphonate
10. Aredia
11. Pamidronate
12. Pamidronate Calcium
13. Pamidronate Monosodium
14. Pamidronic Acid
1. 57248-88-1
2. Aredia
3. Aminomux
4. Disodium Pamidronate
5. Pamidronate Disodium Salt
6. Pamidronic Acid Disodium Salt
7. Cgp 23339a
8. Pamidronate Disodium Anhydrous
9. Pamidronate Disodium Monohydrate
10. Pamidronate (disodium)
11. C7s8vwp5dh
12. Disodium 3-amino-1-hydroxypropane-1,1-diphosphonate
13. Cgp-23339ae
14. Disodium Pamidronate Hydrate
15. Phosphonic Acid, (3-amino-1-hydroxypropylidene)bis-, Disodium Salt
16. Pamidronate (disodium Pentahydrate)
17. Pamedronate Disodium Salt
18. Pamidronatedisodium
19. Pamidronic Acid Sodium Salt
20. Pamindronate Disodium
21. Nsc-722600
22. Cgp 23339 A
23. Einecs 260-647-1
24. Unii-c7s8vwp5dh
25. Sodium 3-amino-1-hydroxypropane-1,1-diylbis(hydrogenphosphonate)
26. Pamidronate Sodium
27. Ahprbp
28. Aminohydroxypropylidene Diphosphonate Disodium Salt
29. (3-amino-1-hydroxypropylidene)diphosphonic Acid Disodium Salt
30. Disodium Dihydrogen (3-amino-1-hydroxypropylidene)bisphosphonate
31. Pamidronate (sodium Salt)
32. Chembl676
33. Schembl18751
34. Dtxsid5042667
35. Pamidronate Disodium Salt Hydrate
36. 3-amino-1-hydroxypropane-1,1-diphosphonic Acid Disodium Salt
37. Cgp-23339a
38. Gcp-23339a
39. Bcp22745
40. Anhydrous Pamidronate Disodium
41. Mfcd00867072
42. S1311
43. Akos015905512
44. Akos025401590
45. Ccg-267232
46. Ig-7913
47. Nsc 722600
48. Cgp 23339a Pound>>gcp23339a
49. Ac-22572
50. Ac-22753
51. Bp164229
52. Pamidronic Acid Disodium Salt [mi]
53. Db-053025
54. Pamidronic Acid Sodium Salt Hydrate- Bio-x
55. D3921
56. Ft-0630698
57. H11402
58. W-105479
59. Q27275302
60. (3-amino-1-hydroxypropylidene)bisphosphonic Acid Disodium
61. Disodium (3-amino-1-hydroxypropane-1,1-diyl)bis[hydrogen (phosphonate)]
62. Disodium Dihydrogen (3-amino-1-hydroxypropylidene)diphosphonate
63. Disodium;[3-amino-1-hydroxy-1-[hydroxy(oxido)phosphoryl]propyl]-hydroxyphosphinate
| Molecular Weight | 279.03 g/mol |
|---|---|
| Molecular Formula | C3H9NNa2O7P2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 278.96496419 g/mol |
| Monoisotopic Mass | 278.96496419 g/mol |
| Topological Polar Surface Area | 167 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 238 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 4 | |
|---|---|
| Drug Name | Aredia |
| Active Ingredient | Pamidronate disodium |
| Dosage Form | Injectable |
| Route | injection; Injection |
| Strength | 30mg/vial; 60mg/vial; 90mg/vial |
| Market Status | Prescription |
| Company | Novartis |
| 2 of 4 | |
|---|---|
| Drug Name | Pamidronate disodium |
| PubMed Health | Pamidronate (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Pamidronate Disodium is a sterile bone-resorption inhibitor available in 30 mg and 90 mg vials for intravenous administration. The pamidronate disodium obtained by combining pamidronic acid and sodium hydroxide is provided in a sterile, ready to use... |
| Active Ingredient | Pamidronate disodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 90mg/vial; 30mg/10ml (3mg/ml); 60mg/10ml (6mg/ml); 30mg/vial; 60mg/vial; 90mg/10ml (9mg/ml) |
| Market Status | Prescription |
| Company | Pliva Lachema; Areva Pharms; Hospira; Teva Pharms Usa; Sun Pharma Global; Fresenius Kabi Usa; Luitpold; Mustafa Nevzat; Eurohlth Intl; Agila Speclts |
| 3 of 4 | |
|---|---|
| Drug Name | Aredia |
| Active Ingredient | Pamidronate disodium |
| Dosage Form | Injectable |
| Route | injection; Injection |
| Strength | 30mg/vial; 60mg/vial; 90mg/vial |
| Market Status | Prescription |
| Company | Novartis |
| 4 of 4 | |
|---|---|
| Drug Name | Pamidronate disodium |
| PubMed Health | Pamidronate (Injection) |
| Drug Classes | Calcium Regulator |
| Drug Label | Pamidronate Disodium is a sterile bone-resorption inhibitor available in 30 mg and 90 mg vials for intravenous administration. The pamidronate disodium obtained by combining pamidronic acid and sodium hydroxide is provided in a sterile, ready to use... |
| Active Ingredient | Pamidronate disodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 90mg/vial; 30mg/10ml (3mg/ml); 60mg/10ml (6mg/ml); 30mg/vial; 60mg/vial; 90mg/10ml (9mg/ml) |
| Market Status | Prescription |
| Company | Pliva Lachema; Areva Pharms; Hospira; Teva Pharms Usa; Sun Pharma Global; Fresenius Kabi Usa; Luitpold; Mustafa Nevzat; Eurohlth Intl; Agila Speclts |
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)


