



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
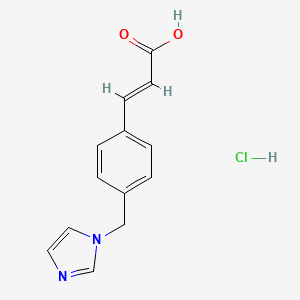

1. 3-(4-(1h-imidazol-1-ylmethyl)phenyl)-2-propenoic Acid
2. 4-(1-imidazoylmethyl)cinnamic Acid
3. Oky 046
4. Oky-046
5. Ozagrel
6. Ozagrel, Monohydrochloride
7. Ozagrel, Monohydrochloride, (e)-isomer
8. Sodium Ozagrel
1. 78712-43-3
2. Ozagrel Hcl
3. Oky 046
4. 74003-18-2
5. Oky-046
6. (e)-3-(4-((1h-imidazol-1-yl)methyl)phenyl)acrylic Acid Hydrochloride
7. Chebi:31954
8. W222u960hs
9. Ozagrel (hydrochloride)
10. (e)-3-[4-(imidazol-1-ylmethyl)phenyl]prop-2-enoic Acid;hydrochloride
11. Dsstox_cid_25506
12. Dsstox_rid_80920
13. Dsstox_gsid_45506
14. Chembl542549
15. (2e)-3-[4-(1h-imidazol-1-ylmethyl)phenyl]-2-propenoic Acid Hydrochloride
16. Smr000469164
17. Sr-01000597793
18. Unii-w222u960hs
19. (2e)-3-(4-(1h-imidazol-1-ylmethyl)phenyl)-2-propenoic Acid Hydrochloride
20. (e)-3-(4-(1h-imidazol-1-ylmethyl)phenyl)-2-propenoic Acid Hydrochloride
21. Oky046 Hydrochloride
22. Cpd000469164
23. Ncgc00016937-01
24. Cas-78712-43-3
25. Oky-046 Hcl
26. Schembl36182
27. Schembl36183
28. Ozagrel Hydrochloride- Bio-x
29. Mls001401435
30. Mls002222319
31. Dtxsid9045506
32. Hy-b0428b
33. Hms1571c19
34. Hms3412f19
35. Hms3676f19
36. Ozagrel Hydrochloride [mi]
37. Bcp09493
38. Tox21_110693
39. S2067
40. Akos015895402
41. Ozagrel Hydrochloride [who-dd]
42. Tox21_110693_1
43. Ccg-100969
44. Nc00219
45. 2-propenoic Acid, 3-(4-(1h-imidazol-1-ylmethyl)phenyl)-, Monohydrochloride, (e)-
46. Ncgc00025195-06
47. Bo164219
48. Ds-11967
49. Ls-14196
50. B2116
51. Cs-0013156
52. O0419
53. Sw197369-4
54. Sr-01000597793-1
55. Sr-01000597793-4
56. Q27114733
57. Trans-4-[(1-imidazolyl)methyl]cinnamic Acid Hydrochloride
58. (e)-3-[4-(imidazol-1-ylmethyl)phenyl]propenoic Acid Hydrochloride
59. (2e)-3-{4-[(1h-imidazol-1-yl)methyl]phenyl}prop-2-enoic Acid Hydrochloride
| Molecular Weight | 264.71 g/mol |
|---|---|
| Molecular Formula | C13H13ClN2O2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 264.0665554 g/mol |
| Monoisotopic Mass | 264.0665554 g/mol |
| Topological Polar Surface Area | 55.1 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 283 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Fibrinolytic Agents
Fibrinolysin or agents that convert plasminogen to FIBRINOLYSIN. (See all compounds classified as Fibrinolytic Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Histamine Antagonists
Drugs that bind to but do not activate histamine receptors, thereby blocking the actions of histamine or histamine agonists. Classical antihistaminics block the histamine H1 receptors only. (See all compounds classified as Histamine Antagonists.)


