



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
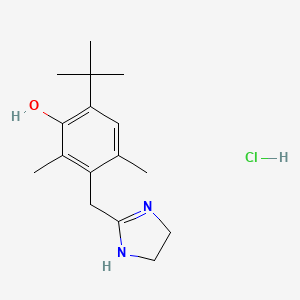

1. Hydrochloride, Oxymetazoline
2. Oxymetazoline
1. 2315-02-8
2. Oxymetazoline Hcl
3. Ocuclear
4. Afrazine
5. Afrin Hydrochloride
6. Sch 9384
7. Rhofade
8. Oxymetazoline (hydrochloride)
9. Visine L.r.
10. Vicks Sinex
11. Nsc-757254
12. K89mj0s5vy
13. 6-tert-butyl-3-(4,5-dihydro-1h-imidazol-2-ylmethyl)-2,4-dimethylphenol;hydrochloride
14. Mls000038040
15. Chebi:7863
16. Agn-199201
17. Sch-9384
18. Smr000059324
19. 2-(4-t-butyl-2,6-dimethyl-3-hydroxybenzyl)-2-imidazolinium Chloride
20. 2,6-dimethyl-2-(4-tertiarybutyl-3-hydroxyphenyl)methylimidazoline Hydrochloride
21. 6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol Monohydrochloride
22. 6-tert-butyl-3-(4,5-dihydro-1h-imidazol-2-ylmethyl)-2,4-dimethylphenol Hydrochloride
23. Phenol, 3-((4,5-dihydro-1h-imidazol-2-yl)methyl)-6-(1,1-dimethylethyl)-2,4-dimethyl-, Monohydrochloride
24. 2-(3-hydroxy-2,6-dimethyl-4-tert-butylbenzyl)-2-imidazoline
25. Lliadine
26. Nasivin
27. Nasivine
28. Neonabel
29. Nostrilla
30. Nafrine Hydrochloride
31. 4-way Nasal Spray
32. Duration Nasal Spray
33. Oxymetazoline Chloride
34. Lliadin Mini Paediatric
35. Sr-01000002705
36. Neo-synephrine 12 Hour
37. Neo-synephrine 12 Hour Ntz
38. Sinex
39. Anefrin Nasal
40. Duration 12 Hour Nasal Spray
41. Benzedrex Nasal Spray 12 Hour
42. Dristan Long Lasting Nasal Mist
43. Sudafed Om
44. Ocuclear (tn)
45. Prestwick_373
46. Einecs 219-015-0
47. St. Joseph Nasal Spray For Children
48. Mfcd00058147
49. Rhfade (tn)
50. Upneeq
51. Opera_id_32
52. 2-(3-hydroxy-2,6-dimethyl-4-t-butylbenzyl)-2-imidazoline Hydrochloride
53. Unii-k89mj0s5vy
54. 3-[(4,5-dihydro-
55. Schembl41247
56. Mls002222207
57. Spectrum1500453
58. Oxymetazoline Hydrochloride,(s)
59. Chembl1200791
60. Hy-b0427a
61. Dtxsid80177729
62. Oximetazoline Hydrochloride
63. Hms1568n09
64. Hms1920d18
65. Pharmakon1600-01500453
66. Tox21_500903
67. Ccg-40216
68. Nsc757254
69. 6-t-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol Hydrochloride
70. Akos000280887
71. Oxymetazoline Hydrochloride (jan/usp)
72. Phenol, 6-t-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethyl-, Hydrochloride
73. Ks-5222
74. Lp00903
75. Nsc 757254
76. Oxymetazoline Hydrochloride [mi]
77. Ncgc00094218-01
78. Ncgc00094218-02
79. Ncgc00094218-03
80. Ncgc00094218-04
81. Ncgc00094218-05
82. Ncgc00261588-01
83. Oxymetazoline Hydrochloride [jan]
84. 2315-02-8 (hcl)
85. Oxymetazoline Hydrochloride [usan]
86. Oxymetazoline Hydrochloride [mart.]
87. Oxymetazoline Hydrochloride [vandf]
88. 4-(2-boc-amino-pyridin-4-yl)-benzoicacid
89. Oxymetazoline Hydrochloride [who-dd]
90. Oxymetazoline Hydrochloride, >=99%, Solid
91. Eu-0100903
92. Ft-0673462
93. O0520
94. Oxymetazoline Hydrochloride [usan:usp:jan]
95. Sw196632-3
96. D01022
97. D91882
98. O 2378
99. Oxymetazoline Hydrochloride, Analytical Standard
100. Oxymetazoline Hydrochloride [orange Book]
101. 315o028
102. A910982
103. Oxymetazoline Hydrochloride [ep Monograph]
104. Oxymetazoline Hydrochloride [usp Impurity]
105. Oxymetazoline Hydrochloride [usp Monograph]
106. Kovanaze Component Oxymetazoline Hydrochloride
107. Sr-01000002705-2
108. Sr-01000002705-4
109. Sr-01000002705-7
110. W-107424
111. Q27107602
112. Oxymetazoline Hydrochloride Component Of Kovanaze
113. Sinex Vapospray Moisturizing 12-hour Decongestant Ultrafine Mist
114. 2,6-dimethyl-4-tertiarybutyl-3-hydroxyphenyl)methylimidazoline Hydrochloride
115. 2-(4-tert-butyl-2,6-dimethyl-3-hydroxybenzyl)-2-imidazolinium Chloride
116. 6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimethylphenol Hydrochloride
117. Oxymetazoline Hydrochloride, European Pharmacopoeia (ep) Reference Standard
118. 1h-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethylphenol Hydrochloride
119. 2-(3-hydroxy-2,6-dimethyl-4-tert-butylbenzyl)-2-imidazoline Hydrochloride
120. 2-(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-4,5-dihydro-1h-imidazol-1-ium Chloride
121. 6-(tert-butyl)-3-((4,5-dihydro-1h-imidazol-2-yl)methyl)-2,4-dimethylphenol Hydrochloride
122. 6-tert-butyl-3-((4,5-dihydro-1h-imidazol-2-yl)methyl)-2,4-dimethylphenol Hydrochloride
123. Oxymetazoline Hydrochloride, Pharmaceutical Secondary Standard; Certified Reference Material
124. Oxymetazoline Hydrochloride, United States Pharmacopeia (usp) Reference Standard
125. 3-[(4,5-dihydro-1h-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethyl-phenol Hydrochloride
| Molecular Weight | 296.83 g/mol |
|---|---|
| Molecular Formula | C16H25ClN2O |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 296.1655411 g/mol |
| Monoisotopic Mass | 296.1655411 g/mol |
| Topological Polar Surface Area | 44.6 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 345 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Nasal Decongestants
Drugs designed to treat inflammation of the nasal passages, generally the result of an infection (more often than not the common cold) or an allergy related condition, e.g., hay fever. The inflammation involves swelling of the mucous membrane that lines the nasal passages and results in inordinate mucus production. The primary class of nasal decongestants are vasoconstrictor agents. (From PharmAssist, The Family Guide to Health and Medicine, 1993) (See all compounds classified as Nasal Decongestants.)
Sympathomimetics
Drugs that mimic the effects of stimulating postganglionic adrenergic sympathetic nerves. Included here are drugs that directly stimulate adrenergic receptors and drugs that act indirectly by provoking the release of adrenergic transmitters. (See all compounds classified as Sympathomimetics.)
Adrenergic alpha-Agonists
Drugs that selectively bind to and activate alpha adrenergic receptors. (See all compounds classified as Adrenergic alpha-Agonists.)






