



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
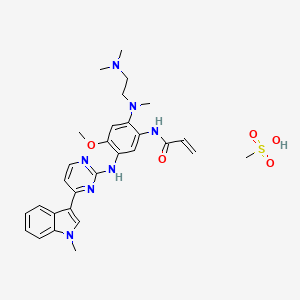

1. Azd-9291
2. Azd-9291 Mesylate
3. Azd9291
4. Azd9291 Mesylate
5. Mereletinib
6. Mereletinib Mesilate
7. Mereletinib Mesylate
8. N-(2-((2-(dimethylamino)ethyl)methylamino)-4-methoxy-5-((4-(1-methyl-1h-indol-3-yl)-2-pyrimidinyl)amino)phenyl)-2-propenamide
9. N-(2-((2-(dimethylamino)ethyl)methylamino)-4-methoxy-5-((4-(1-methyl-1h-indol-3-yl)-2-pyrimidinyl)amino)phenyl)-2-propenamide Methanesulfonate (1:1)
10. Osimertinib
11. Osimertinib Mesilate
12. Tagrisso
1. 1421373-66-1
2. Azd-9291 Mesylate
3. Azd9291 Mesylate
4. Azd-9291 (mesylate)
5. Mereletinib Mesylate
6. Tagrisso
7. Osimertinib Mesilate
8. Mereletinib Mesilate
9. Osimertinib Mesylate [usan]
10. Rdl94r2a16
11. N-[2-[2-(dimethylamino)ethyl-methylamino]-4-methoxy-5-[[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino]phenyl]prop-2-enamide;methanesulfonic Acid
12. N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(1-methyl-1h-indol-3-yl)pyrimidin-2-yl)amino)phenyl)acrylamide Methanesulfonate
13. Osimertinib Mesilate (jan)
14. Osimertinib Mesylate (usan)
15. 2-propenamide, N-(2-((2-(dimethylamino)ethyl)methylamino)-4-methoxy-5-((4-(1-methyl-1h-indol-3-yl)-2-pyrimidinyl)amino)phenyl)-, Methanesulfonate (1:1)
16. Osimertinib Mesilate [jan]
17. 2-propenamide, N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1h-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-, Compd. With Methanesulfonate (1:2)
18. Unii-rdl94r2a16
19. Tagrisso (tn)
20. Osimertinib Monomesylate
21. Azd 9291 Mesylate
22. Osimertinib Methanesulfonate
23. Amy226
24. Chembl3545063
25. Schembl14661152
26. Chebi:90948
27. Osimertinib Mesylate [mi]
28. Dtxsid101027822
29. Bcp09934
30. Ex-a1577
31. Hy-15772a
32. Mfcd28137994
33. Osimertinib Mesilate [who-dd]
34. Akos026673944
35. Ds-9913
36. Sb22953
37. Ac-29022
38. Da-35303
39. Osimertinib Mesylate [orange Book]
40. Azd-9291 Mesylate (osimertinibmereletinib)
41. Ft-0699962
42. S5078
43. D10766
44. Q27162942
45. Azd9291 Ms Salt, Osimertinib Ms Salt; Mereletinib Ms Salt
46. N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1h-indol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide Methanesulfonate
47. N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1h-indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-propenamide Methanesulfonate
1. Osimertinib
| Molecular Weight | 595.7 g/mol |
|---|---|
| Molecular Formula | C29H37N7O5S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 10 |
| Exact Mass | 595.25768848 g/mol |
| Monoisotopic Mass | 595.25768848 g/mol |
| Topological Polar Surface Area | 150 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 845 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
TAGRISSO as monotherapy is indicated for:
-the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations
- the first-line treatment of adult patients NSCLC with activating EGFR mutations.
- the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC.
TAGRISSO as monotherapy is indicated for:
- the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA non-small cell lung cancer (NSCLC) whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.
- the first-line treatment of adult patients with locally advanced or metastatic NSCLC with activating EGFR mutations.
- the treatment of adult patients with locally advanced or metastatic EGFR T790M mutation-positive NSCLC.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01XE


