



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
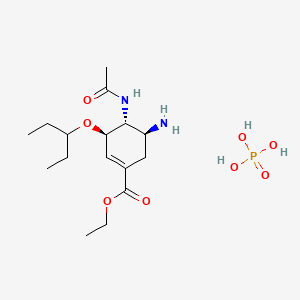

1. Gs 4071
2. Gs 4104
3. Gs-4071
4. Gs-4104
5. Gs4071
6. Gs4104
7. Oseltamivir
8. Tamiflu
1. 204255-11-8
2. Tamiflu
3. Oseltamivir (phosphate)
4. Oseltamir Phosphate
5. Agucort
6. Ro 64-0796/002
7. Aseltamivir Phosphate
8. Gs 4104
9. Oseltamivir (as Phosphate)
10. 4a3o49ngez
11. Ethyl (3r,4r,5s)-4-acetamido-5-amino-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate Phosphate
12. Chebi:7799
13. Ethyl (3r,4r,5s)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate Phosphate (1:1)
14. (3r,4r,5s)-ethyl 4-acetamido-5-amino-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate Phosphate
15. (3r-(3alpha,4beta,5alpha))-ethyl 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate Phosphate (1:1)
16. Ro-64-0796-002
17. Ro-64-0796/002
18. 1-cyclohexene-1-carboxylic Acid, 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-, Ethyl Ester, (3r,4r,5s)-, Phosphate (1:1)
19. Phosphoric Acid Ethyl (3r,4r,5s)-5-amino-4-acetamido-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate
20. Oseltamivir Phosphate [usan]
21. Unii-4a3o49ngez
22. Sr-05000001499
23. Mfcd08059548
24. Ebilfumin (tn)
25. Oseltamivir Phosphate [usan:usp]
26. Ethyl (3r,4r,5s)-4-acetamido-5-amino-3-pentan-3-yloxycyclohexene-1-carboxylate;phosphoric Acid
27. Tamiflu (tn)
28. Oseltamiviri Phosphas
29. Gs 4104 Phosphate
30. Schembl8730
31. (3r-(3alpha,4beta,5alpha)-ethyl 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate Phosphate (1:1)
32. Mls006011559
33. Amy583
34. Chembl1200340
35. Dtxsid0044230
36. Oseltamivir Phosphate (jan/usp)
37. Bcpp000138
38. Oseltamivir Phosphate [mi]
39. Oseltamivir Phosphate [jan]
40. Act03369
41. Oseltamivir Phosphate [vandf]
42. S2597
43. Oseltamivir Phosphate [mart.]
44. Akos015896056
45. Oseltamivir Phosphate [usp-rs]
46. Oseltamivir Phosphate [who-dd]
47. Oseltamivir Phosphate [who-ip]
48. Bcp9001033
49. Ccg-230250
50. Cs-0871
51. Ks-1184
52. Nsc 758897
53. Oseltamivir Phosphate, >=98% (hplc)
54. 1-cyclohexene-1-carboxylic Acid, 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-, Ethyl Ester, (3r-(3alpha,4beta,5alpha)-, Phosphate (1:1)
55. Ac-25911
56. Bo164181
57. Ethyl (3r,4r,5s)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate, Phosphate (1:1)
58. Hy-17016
59. Smr004703323
60. Oseltamivir Phosphate [orange Book]
61. Oseltamivir Phosphate [ep Monograph]
62. Oseltamivir Phosphate [usp Impurity]
63. Oseltamivir Phosphate [usp Monograph]
64. Oseltamiviri Phosphas [who-ip Latin]
65. Gs-4104/002
66. C08093
67. D00900
68. 255o118
69. Sr-05000001960
70. J-013302
71. J-523838
72. Sr-05000001499-2
73. Sr-05000001960-1
74. Q27107588
75. Z1550675456
76. Benzofuran-3-yl-(3-boc-amino-azetidin-1-yl)-aceticacid
77. Oseltamivir Phosphate, United States Pharmacopeia (usp) Reference Standard
78. Oseltamivir Phosphate (impurity B Free), European Pharmacopoeia (ep) Reference Standard
79. Oseltamivir Phosphate, Pharmaceutical Secondary Standard; Certified Reference Material
80. (3r,4r,5s)-4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic Acid Ethyl Ester Phosphate
81. (3r,4r,5s)-4-acetylamino-5-amino-3-(1-ethyl-propoxy)-cyclohex-1-enecarboxylic Acid Ethyl Ester Phosphate
82. (3r,4r,5s)-4-acetylamino-5-amino-3-(1-ethyl-propoxy)-cyclohex-1-enecarboxylic Acid Ethyl Ester Phosphoric Acid Salt
83. (3r,5s)-ethyl 4-acetamido-5-amino-3-(pentan-3-yloxy)cyclohex-1-enecarboxylate Phosphate;osteltamivir Phosphate
84. (3r-(3.alpha.,4.beta.,5.alpha.))-ethyl 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate Phosphate (1:1)
85. Ethyl (3r,4r,5s)-5-amino-4-acetamido-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylate; Phosphoric Acid
86. Ethyl-(3r,4r,5s)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate Phosphate
| Molecular Weight | 410.40 g/mol |
|---|---|
| Molecular Formula | C16H31N2O8P |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 8 |
| Exact Mass | 410.18180295 g/mol |
| Monoisotopic Mass | 410.18180295 g/mol |
| Topological Polar Surface Area | 168 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 468 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Oseltamivir phosphate |
| PubMed Health | Oseltamivir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | TAMIFLU (oseltamivir phosphate) is available as capsules containing 30 mg, 45 mg, or 75 mg oseltamivir for oral use, in the form of oseltamivir phosphate, and as a powder for oral suspension, which when constituted with water as directed contains 6 m... |
| Active Ingredient | Oseltamivir phosphate |
| Dosage Form | Capsule |
| Route | oral |
| Strength | 30mg |
| Market Status | Tentative Approval |
| Company | Natco Pharma |
| 2 of 4 | |
|---|---|
| Drug Name | Tamiflu |
| Drug Label | TAMIFLU (oseltamivir phosphate) is available as capsules containing 30 mg, 45 mg, or 75 mg oseltamivir for oral use, in the form of oseltamivir phosphate, and as a powder for oral suspension, which when constituted with water as directed contains 6 m... |
| Active Ingredient | Oseltamivir phosphate |
| Dosage Form | Capsule; For suspension |
| Route | Oral |
| Strength | eq 75mg base; eq 6mg base/ml; eq 30mg base; eq 45mg base |
| Market Status | Prescription |
| Company | Roche |
| 3 of 4 | |
|---|---|
| Drug Name | Oseltamivir phosphate |
| PubMed Health | Oseltamivir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | TAMIFLU (oseltamivir phosphate) is available as capsules containing 30 mg, 45 mg, or 75 mg oseltamivir for oral use, in the form of oseltamivir phosphate, and as a powder for oral suspension, which when constituted with water as directed contains 6 m... |
| Active Ingredient | Oseltamivir phosphate |
| Dosage Form | Capsule |
| Route | oral |
| Strength | 30mg |
| Market Status | Tentative Approval |
| Company | Natco Pharma |
| 4 of 4 | |
|---|---|
| Drug Name | Tamiflu |
| Drug Label | TAMIFLU (oseltamivir phosphate) is available as capsules containing 30 mg, 45 mg, or 75 mg oseltamivir for oral use, in the form of oseltamivir phosphate, and as a powder for oral suspension, which when constituted with water as directed contains 6 m... |
| Active Ingredient | Oseltamivir phosphate |
| Dosage Form | Capsule; For suspension |
| Route | Oral |
| Strength | eq 75mg base; eq 6mg base/ml; eq 30mg base; eq 45mg base |
| Market Status | Prescription |
| Company | Roche |
* Treatment of influenza:
Tamiflu is indicated in adults and children including full term neonates who present with symptoms typical of influenza, when influenza virus is circulating in the community. Efficacy has been demonstrated when treatment is initiated within two days of first onset of symptoms. Prevention of influenza
- Post-exposure prevention in individuals one year of age or older following contact with a clinically diagnosed influenza case when influenza virus is circulating in the community.
- The appropriate use of Tamiflu for prevention of influenza should be determined on a case-by-case basis by the circumstances and the population requiring protection. In exceptional situations (e. g. in case of a mismatch between the circulating and vaccine virus strains, and a pandemic situation) seasonal prevention could be considered in individuals one year of age or older.
- Tamiflu is indicated for post-exposure prevention of influenza in infants less than 1 year of age during a pandemic-influenza outbreak.
Tamiflu is not a substitute for influenza vaccination.
The use of antivirals for the treatment and prevention of influenza should be determined on the basis of official recommendations. Decisions regarding the use of oseltamivir for treatment and prophylaxis should take into consideration what is known about the characteristics of the circulating influenza viruses, available information on influenza drug susceptibility patterns for each season and the impact of the disease in different geographical areas and patient populations.
Treatment and prevention of influenza
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J05AH02








