


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
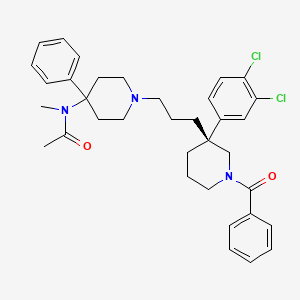

1. (s)-(n)-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl)propyl)-4-phenylpiperidin-4-yl)-n-methylacetamide
2. Sr 142801
3. Sr 142806
4. Sr-142801
5. Sr142801
1. 160492-56-8
2. Osanetant [inn]
3. Sr142801
4. Sr-142801
5. Sb-236984
6. Sr-14280
7. Sr-142806
8. Chembl346178
9. K7g81n94dt
10. N-(1-(3-((r)-1-benzoyl-3-(3,4-dichlorophenyl)-3-piperidyl)propyl)-4-phenyl-4-piperidyl)-n-methylacetamide
11. N-(1-(3-((3r)-1-benzoyl-3-(3,4-dichlorophenyl)-3-piperidinyl)propyl)-4-phenyl-4-piperidinyl)-n-methylacetamide
12. Sr 142801
13. [3h]osanetant
14. (r)-n-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl)propyl)-4-phenylpiperidin-4-yl)-n-methylacetamide
15. Acetamide, N-[1-[3-[(3r)-1-benzoyl-3-(3,4-dichlorophenyl)-3-piperidinyl]propyl]-4-phenyl-4-piperidinyl]-n-methyl-
16. N-(1-{3-[(r)-1-benzoyl-3-(3,4-dichloro-phenyl)-piperidin-3-yl]-propyl}-4-phenyl-piperidin-4-yl)-n-methyl-acetamide
17. Sr 142806
18. Unii-k7g81n94dt
19. N-[1-[3-[(3r)-1-(benzoyl)-3-(3,4-dichlorophenyl)piperidin-3-yl]propyl]-4-phenylpiperidin-4-yl]-n-methylacetamide
20. N-[1-[3-[(3r)-1-benzoyl-3-(3,4-dichlorophenyl)-3-piperidinyl]propyl]-4-phenyl-4-piperidinyl]-n-methylacetamide
21. [3h]sr142801
22. [3h]sr142,801
23. Mls006010325
24. N-[1-[3-[(3r)-1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl]propyl]-4-phenylpiperidin-4-yl]-n-methylacetamide
25. Schembl119440
26. Gtpl2110
27. Gtpl3480
28. Dtxsid901027521
29. Zinc3935475
30. Bdbm50291261
31. Pdsp1_001495
32. Pdsp2_001479
33. Akos015895948
34. Bcp9001032
35. Db04872
36. Ncgc00263110-01
37. Ncgc00263110-02
38. (s)-(n)-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl)propyl)-4-phenylpiperidin-4-yl)-n-methylacetamide
39. Acetamide, N-(1-(3-(1-benzoyl-3-(3,4-dichlorophenyl)-3-piperidinyl)propyl)-4-phenyl-4-piperidinyl)-n-methyl-, (r)-
40. Hy-14551
41. Smr004701389
42. Cs-0003438
43. Sr 142,806
44. Sr-142,801
45. E98685
46. Q7105703
47. Brd-k15646852-001-01-5
48. (s)-(+)-n-((3-[1-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl]prop-1-yl)-4-phenylpiperidin-4-yl)-n-methylacetamine
| Molecular Weight | 606.6 g/mol |
|---|---|
| Molecular Formula | C35H41Cl2N3O2 |
| XLogP3 | 7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 8 |
| Exact Mass | 605.2575829 g/mol |
| Monoisotopic Mass | 605.2575829 g/mol |
| Topological Polar Surface Area | 43.9 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 897 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Potential therapy for schizophrenia, depression and visceral pain.
Osanetant is a neurokinin-3 (NK3) receptor antagonist. Preliminary clinical trials have demonstrated that osanetant is superior to placebo on global assessment of efficacy and measures of positive symptoms in schizophrenia.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
The mechanism of action of osanetant is uncertain at this point. Various preclinical data indicate that activation of NK3 receptors enhances the release of biogenic amines, including dopamine and serotonin. NK3 receptor antagonists could block NK3-receptor-mediated activation of these systems.


