


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
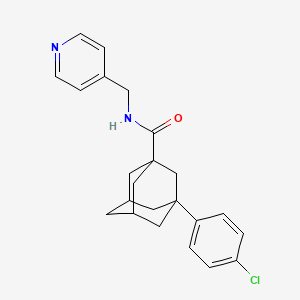

1. 3-(4-chlorophenyl)-adamantane-1-carboxylic Acid (pyridin-4-ylmethyl)amide
2. 4-pyridinylmethyl-3-(4-chlorophenyl) Adamantane Carboxamide
3. Abc 294640
4. Abc-294640
5. Abc294640
1. Abc294640
2. Opaganib
3. 915385-81-8
4. Abc-294640
5. Abc 294640
6. Chembl2158685
7. 4-pyridinylmethyl-3-(4-chlorophenyl) Adamantane Carboxamide
8. Drg21oq517
9. Yeliva
10. Tricyclo(3.3.1.13,7)decane-1-carboxamide, 3-(4-chlorophenyl)-n-(4-pyridinylmethyl)-
11. Abc294640opaganib
12. Opaganib [inn]
13. Opaganib [who-dd]
14. Opaganib (abc294640)
15. Gtpl6624
16. Schembl1548333
17. Chebi:124965
18. Dtxsid801318727
19. Hms3402p05
20. Amy42174
21. Bcp08959
22. Ex-a1962
23. Bdbm50393642
24. Nsc796101
25. Akos027327311
26. Ccg-268417
27. Cs-0877
28. Db12764
29. Nsc-796101
30. Sb17167
31. Ac-33116
32. As-56117
33. Ba162479
34. Hy-16015
35. S7174
36. Brd-a70814879-003-01-8
37. Q27074100
38. Abc294640
39. 915385-81-8
40. Abc-294640
41. 4-pyridinylmethyl 3(4-chlorophenyl)adamantine Carboxamide
42. (1s,3r,5r,7s)-3-(4-chlorophenyl)-n-(pyridin-4-ylmethyl)adamantane-1-carboxamide
| Molecular Weight | 380.9 g/mol |
|---|---|
| Molecular Formula | C23H25ClN2O |
| XLogP3 | 4.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 380.1655411 g/mol |
| Monoisotopic Mass | 380.1655411 g/mol |
| Topological Polar Surface Area | 42 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 551 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Opaganib selectively inhibits [sphingosine kinase-2 (SK2)](https://go.drugbank.com/polypeptides/Q9NRA0). This inhibition blocks the synthesis of sphingosine 1-phosphate (S1P) and its activities (which includes regulation of fundamental biological processes such as cell proliferation, migration, immune cell trafficking, angiogenesis, immune-modulation, and suppression of innate immune responses from T-cells). This drug has dual anti-inflammatory and antiviral activity targeting a host cell component and is unaffected by viral mutation, contributing to minimization of the likelihood of resistance. It is currently being investigated against COVID-19 as it has demonstrated anti-SARS-CoV-2 activity in studies.


