
Synopsis
0
KDMF
0
VMF
0
Australia
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
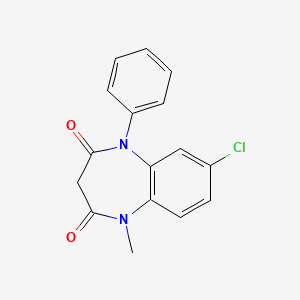

1. 1-phenyl-5-methyl-8-chloro-1,2,4,5- Tetrahydro-2,4-diketo-3h-1,5-benzodiazepine
2. Frisium
3. Hr 376
4. Lm 2717
5. Lm-2717
6. Lm2717
7. Onfi
8. Urbanyl
1. Frisium
2. Urbanyl
3. Chlorepin
4. Clorepin
5. Urbadan
6. 22316-47-8
7. Onfi
8. Lm-2717
9. Hr 376
10. Clobazamum
11. Mystan
12. Lm 2717
13. H-4723
14. Ru-4723
15. H 4723
16. 1h-1,5-benzodiazepine-2,4(3h,5h)-dione, 7-chloro-1-methyl-5-phenyl-
17. 7-chloro-1-methyl-5-phenyl-1h-1,5-benzodiazepine-2,4(3h,5h)-dione
18. Hr-376
19. 7-chloro-1-methyl-5-phenyl-1,5-benzodiazepine-2,4-dione
20. Nsc 336279
21. 1-phenyl-5-methyl-8-chloro-1,2,4,5-tetrahydro-2,4-dioxo-3h-1,5-benzodiazepine
22. Chebi:31413
23. 7-chloro-1-methyl-5-phenyl-1h-1,5-benzodiazepine-2,4-(3h,5h)-dione
24. Nsc-336279
25. 2mro291b4u
26. Nsc336279
27. Ncgc00168249-01
28. Caastilium
29. Noiafren
30. Urbanil
31. Odipam
32. Clobazamum [inn-latin]
33. Clobazepam
34. 7-chloro-1-methyl-5-phenyl-1,5-dihydro-benzo[b][1,4]diazepine-2,4-dione
35. 7-chloro-1-methyl-5-phenyl-2,3,4,5-tetrahydro-1h-1,5-benzodiazepine-2,4-dione
36. Mystan (tn)
37. Ccris 7506
38. Einecs 244-908-7
39. Brn 0758410
40. Unii-2mro291b4u
41. Clobazam (jan/usan/inn)
42. Clobazam [usan:inn:ban]
43. Colbazam
44. Perizam
45. Tapclob
46. Urbanol
47. Dea No. 2751
48. Sympazan
49. Onfi (tn)
50. Clobazam [usan]
51. Clobazam [inn]
52. Clobazam [jan]
53. Clobazam [mi]
54. Clobazam [vandf]
55. Colbazam [vandf]
56. Clobazam [mart.]
57. Clobazam [who-dd]
58. 1-phenyl-5-methyl-8-chloro-1,2,4,5-tetrahydro-2,4-diketo-3h-1,5-benzodiazepine
59. Dsstox_cid_26759
60. Dsstox_rid_81883
61. Dsstox_gsid_46759
62. Schembl43038
63. 5-24-08-00034 (beilstein Handbook Reference)
64. Mls003899217
65. Chembl70418
66. Clobazam [orange Book]
67. Gtpl7149
68. Zinc1175
69. Clobazam [ep Monograph]
70. Dtxsid2046759
71. Hsdb 8343
72. Clobazam 0.1 Mg/ml In Methanol
73. Clobazam 1.0 Mg/ml In Methanol
74. Hy-a0041
75. Tox21_112608
76. Bdbm50247888
77. Akos025401921
78. Cs-6756
79. Db00349
80. Smr000058811
81. Wln: T67 Gnv Jvn Ihj Cg G1 Kr
82. Cas-22316-47-8
83. Db-045870
84. C-2991
85. D01253
86. Q412164
87. Sr-01000937603
88. Sr-01000937603-2
89. 7-chloro-1-methyl-5-phenyl-1h-1,4(3h,5h)-dione
90. 7-chloro-1-methyl-5-phenyl-1h-1,4-(3h,5h)-dione
91. 1h-1,4(3h,5h)-dione, 7-chloro-1-methyl-5-phenyl-
92. 7-chloro-1-methyl-5-phenyl-1,5-benzodiazepine-2,4(3h)-dione
93. 1-phenyl 5-methyl 8-chloro 1,2,4,5-tetrahydo 2,4-dioxo 3h-1,5-benzodiazepine
94. 1-phenyl 5-methyl 8-chloro 1,2,4,5-tetrahydro 2,4-dioxo 3h-1,5-benzodiazepine
95. 1-phenyl-5-methyl-8-chloro-1,2,4,5-tetrahydro-2,4-dioxo-3h-1, 5-benzodiazepine
96. 1-phenyl-5-methyl-8-chloro-1,4,5-tetrahydro-2,4-dioxo-3h-1,5-benzodiazepine
97. Clobazam Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 300.74 g/mol |
|---|---|
| Molecular Formula | C16H13ClN2O2 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 1 |
| Exact Mass | 300.0665554 g/mol |
| Monoisotopic Mass | 300.0665554 g/mol |
| Topological Polar Surface Area | 40.6 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 423 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Onfi |
| PubMed Health | Clobazam (By mouth) |
| Drug Classes | Anticonvulsant, Sedative-Hypnotic |
| Drug Label | Table 4. Description Proprietary Name: ONFI Established Name: Clobazam Dosage Forms: Tablet and Oral Suspension Route of Administration: Oral Established Pharmacologic Class of Drug: Benzodiazepine Chemical Name:... |
| Active Ingredient | Clobazam |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 2.5mg/ml; 10mg; 20mg |
| Market Status | Prescription |
| Company | Lundbeck |
| 2 of 2 | |
|---|---|
| Drug Name | Onfi |
| PubMed Health | Clobazam (By mouth) |
| Drug Classes | Anticonvulsant, Sedative-Hypnotic |
| Drug Label | Table 4. Description Proprietary Name: ONFI Established Name: Clobazam Dosage Forms: Tablet and Oral Suspension Route of Administration: Oral Established Pharmacologic Class of Drug: Benzodiazepine Chemical Name:... |
| Active Ingredient | Clobazam |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 2.5mg/ml; 10mg; 20mg |
| Market Status | Prescription |
| Company | Lundbeck |
Anticonvulsants
National Library of Medicine's Medical Subject Headings. Clobazam. Online file (MeSH, 2016). Available from, as of June 24, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Clobazam is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 6, 2016: https://clinicaltrials.gov/search/intervention=CLOBAZAM
Onfi (clobazam) is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older. /Included in US product label/
NIH; DailyMed. Current Medication Information for Onfi (Clobazam) Tablet; Onfi (Clobazam) Suspension (Updated: December 2014). Available from, as of July 1, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de03bd69-2dca-459c-93b4-541fd3e9571c
Clobazam currently is FDA-labeled only for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome. However, the drug has demonstrated broad anticonvulsant activity and has been used extensively in adults and pediatric patients with a wide range of other seizure disorders, which have sometimes been refractory, including partial, generalized, and myoclonic seizures. /NOT included in US product label/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2294
Clobazam has been used in the treatment of anxiety disorders and has been labeled for the short-term (2-4 weeks) treatment of anxiety in some countries outside the US. However, the drug currently is not FDA-labeled for the treatment of anxiety disorders in the US. /NOT included in US product label/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2294
Since clobazam has a CNS depressant effect, patients or their caregivers should be cautioned against concomitant use of other CNS depressant drugs or alcohol, and cautioned that the effects of other CNS depressants or alcohol may be potentiated.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2295
Somnolence and sedation are among the most common adverse effects associated with clobazam therapy. These effects generally begin within the first month of treatment and may diminish with continued therapy. In a placebo-controlled study of patients with Lennox-Gastaut syndrome, somnolence or sedation was reported in 26% of patients who received clobazam compared with 15% of those who received placebo. Somnolence and sedation were observed at all effective dosages in clinical trials and were dose related. The sedative effect of clobazam is reportedly less pronounced than that of other commercially available benzodiazepines
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2295
Withdrawal symptoms (e.g., convulsions, psychosis, hallucinations, behavioral disorders, tremor, anxiety, irritability, dysphoria, insomnia, headache, palpitations, diarrhea) have been reported following abrupt discontinuance of benzodiazepines, including clobazam; the risk of withdrawal symptoms is greater with increasing dosage and duration of treatment. Such withdrawal reactions occur in patients who have developed physical dependence on these drugs. More severe withdrawal symptoms usually are limited to patients who abruptly discontinue therapy after receiving excessive dosages of benzodiazepines for an extended period of time. Milder withdrawal symptoms (e.g., dysphoria, anxiety, insomnia) generally have been reported following abrupt discontinuance of benzodiazepines in patients continuously receiving therapeutic dosages for several months.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2295
Abrupt discontinuance and rapid dosage reduction of clobazam should be avoided to minimize the risk of precipitating or exacerbating seizures, status epilepticus, and withdrawal symptoms. When therapy is discontinued, dosage of the drug should be decreased gradually (i.e., by 5-10 mg daily at weekly intervals).
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2295
For more Drug Warnings (Complete) data for Clobazam (21 total), please visit the HSDB record page.
For treatment and management of epilepsy and seizures associated with Lennox-Gastaut syndrome, a difficult-to-treat form of childhood epilepsy.
FDA Label
Similar to other benzodiazepines, clobazam binds to the interface of the and 2-subunit of the GABA-A receptor. However, it is considered a partial agonist to GABA-A receptors which sets clobazam apart from 1,4-benzodiazepines which are full agonist. The significance of this difference is that one may experience less sedation with clobazam than with other benzodiazepines. Unlike the endogenous GABA ligand, clobazam binds allosterically to the GABA receptor to increase the frequency of the chloride channel opening and membrane permeability to chloride ions. Pharmacodynamic tolerance has been demonstrated in animal models.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
GABA-A Receptor Agonists
Endogenous compounds and drugs that bind to and activate GABA-A RECEPTORS. (See all compounds classified as GABA-A Receptor Agonists.)
N05BA09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA09 - Clobazam
Absorption
After oral administration of clobazam, it is almost completely absorbed (87% of dose). Bioavailability relative to solution was almost at 100%. Food does not affect absorption. Tmax = 1-3 hours.
Route of Elimination
Clobazam is eliminated via the urine (~94%) as metabolites.
Volume of Distribution
Vdss = 100 L. This high volume of distribution suggests extensive distribution to body tissues.
Clearance
Median estimated clearance = 2.49 L/h
/MILK/ Onfi is excreted in human milk.
NIH; DailyMed. Current Medication Information for Onfi (Clobazam) Tablet; Onfi (Clobazam) Suspension (Updated: December 2014). Available from, as of July 1, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de03bd69-2dca-459c-93b4-541fd3e9571c
/MILK/ After oral administration of (14)C-clobazam (NH-15,7- chloro-1-methyl-5-phenyl-1H-1,5-benzodiazepine-2,4-(3H,5H)-dione) (4 mg/kg) to pregnant and lactating rats, the placental transfer and the secretion of radioactivity into milk were studied. Whole body autoradiograms of pregnant rats showed that radioactivity was distributed to the whole body of the fetus. Concentrations of radioactivity in fetal brain and blood were lower than the maternal plasma and placental levels, and decreased rapidly. The extent of transfer of radioactivity into the fetus reached maximum at 30 min after administration, amounting to 0.10% of the radioactivity administered per fetus. The composition of clobazam and its metabolites in plasma of pregnant rats at 30 min after oral administration was similar to that of non-pregnant rats. Concentration of radioactivity in the milk was 1.4 times higher than that in the blood, reached maximum 30 min after administration, then declined rapidly. The radioactivity transferred to the suckling via milk reached a maximum of 0.023% of the dose up to 8 hr after administration. Excretion of radioactivity from the suckling was slow. The composition of clobazam and its metabolites in aggregated milk in the suckling stomach was similar to that of female rat plasma.
Yokoyama N et al; Iyakuhin Kenkyu 25 (8): 640-7 (1994)
Clobazam is lipophilic and distributes rapidly throughout the body. The apparent volume of distribution at steady state was approximately 100 L. The in vitro plasma protein binding of clobazam and N-desmethylclobazam is approximately 80-90% and 70%, respectively.
NIH; DailyMed. Current Medication Information for Onfi (Clobazam) Tablet; Onfi (Clobazam) Suspension (Updated: December 2014). Available from, as of July 1, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de03bd69-2dca-459c-93b4-541fd3e9571c
Clobazam is rapidly and extensively absorbed following oral administration. The time to peak concentrations (Tmax) of clobazam tablets under fasted conditions ranged from 0.5 to 4 hours after single- or multiple-dose administrations. The relative bioavailability of clobazam tablets compared to an oral solution is approximately 100%. After single dose administration of the oral suspension under fasted conditions, the Tmax ranged from 0.5 to 2 hours. Based on exposure (Cmax and AUC) of clobazam, Onfi tablets and suspension were shown to have similar bioavailability under fasted conditions. The administration of Onfi tablets with food or when crushed in applesauce does not affect absorption. Although not studied, the oral bioavailability of the oral suspension is unlikely to be affected under fed conditions.
NIH; DailyMed. Current Medication Information for Onfi (Clobazam) Tablet; Onfi (Clobazam) Suspension (Updated: December 2014). Available from, as of July 1, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de03bd69-2dca-459c-93b4-541fd3e9571c
Clobazam is extensively metabolized in the liver via N-demethylation and hydroxylation to form two major metabolites, N-desmethylclobazam (norclobazam) and 4'-hydroxyclobazam, respectively. N-desmethylclobazam (norclobazam) retains pharmacological activity. Norclobazam is one-fourth the potency of clobazam. The main enzyme that facilitates the process of N-demethylation is CYP3A4, and to a lesser extent by CYP2C19 and CYP2B6. Norclobazam itself is also metabolized via hydroxylation, primarily by CYP2C19. The formation of 4'-hydroxyclobazam is facilitated by CYP2C18 and CYP2C19. A factor in determining extent of metabolism is the genetic profile of the individual patient as CYP2C19 is a polymorphic enzyme.
The polymorphic CYP2C19 is the major contributor to the metabolism of the pharmacologically active N-desmethylclobazam. In CYP2C19 poor metabolizers, levels of N-desmethylclobazam were 5-fold higher in plasma and 2- to 3-fold higher in the urine than in CYP2C19 extensive metabolizers.
NIH; DailyMed. Current Medication Information for Onfi (Clobazam) Tablet; Onfi (Clobazam) Suspension (Updated: December 2014). Available from, as of July 1, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de03bd69-2dca-459c-93b4-541fd3e9571c
Clobazam is extensively metabolized in the liver, with approximately 2% of the dose recovered in urine and 1% in feces as unchanged drug. The major metabolic pathway of clobazam involves N-demethylation, primarily by CYP3A4 and to a lesser extent by CYP2C19 and CYP2B6. N-desmethylclobazam, an active metabolite, is the major circulating metabolite in humans, and at therapeutic doses, plasma concentrations are 3-5 times higher than those of the parent compound. Based on animal and in vitro receptor binding data, estimates of the relative potency of N-desmethylclobazam compared to parent compound range from 1/5 to equal potency. N-desmethylclobazam is extensively metabolized, mainly by CYP2C19. N-desmethylclobazam and its metabolites comprise approximately 94% of the total drug-related components in urine. Following a single oral dose of radiolabeled drug, approximately 11% of the dose was excreted in the feces and approximately 82% was excreted in the urine.
NIH; DailyMed. Current Medication Information for Onfi (Clobazam) Tablet; Onfi (Clobazam) Suspension (Updated: December 2014). Available from, as of July 1, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de03bd69-2dca-459c-93b4-541fd3e9571c
A four-year-old male with symptomatic generalized epilepsy presented with ataxia, eye rolling, and episodes of back arching which were of non-epileptic origin following the introduction of clobazam at 0.75 mg/kg/day. Concurrent antiepileptic medication was lamotrigine at 13 mg/kg/day. Clobazam plasma levels were within the normal range, while N-desmethylclobazam (DCLB) concentrations were between five and seven times above the upper limit of the normal range. The plasma elimination half-life for DCLB was prolonged, suggesting a genetic variability in DCLB metabolism leading to toxicity. Reduction in the dose of clobazam to 0.3 mg/kg/day was associated with resolution of the non-epileptic neurological symptoms, reduction in DCLB plasma levels, and maintenance of seizure control.
PMID:16780634 Aylett SE et al; Dev Med Child Neurol 48 (7): 612-5 (2006)
Clobazam has known human metabolites that include 4-Hydroxyclobazam and N-desmethylclobazam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The mean elimination half life of an oral dose of clobazam 40 mg is 32 hours. It's main metabolite, norclobazam, as a half life of 57 hours. The half life in adult patients with epilepsy are higher than those that are healthy.
The mean elimination half-life of clobazam is approximately 36-42 hours and the mean elimination half-life of N-desmethylclobazam is approximately 71-82 hours.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2297
Clobazam binds at distinct binding sites associated with the chloride ionopore at the post-synaptic GABA receptor. These GABA receptors are in various locations in the CNS (limbic, reticular formation) and clobazam increases the duration of time for which the chloride ionopore is open. As a result, hyper polarization and stabilization of the membrane occur as the post-synaptic inhibitory effect of GABA is enhanced.
About the Company : EUROAPI focuses on reinventing active ingredient solutions to sustainably meet the needs of customers and patients worldwide. The company is a leading API player with a portfolio o...
 Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
Honour is a leading global CDMO and specialty chemicals manufacturer with seven world-class sites delivering quality-driven solutions.
About the Company : Honour is a leading global CDMO and trusted manufacturer of specialty chemicals and ingredients, with seven world-class facilities meeting global safety and quality standards. Thro...
About the Company : CALYX is a manufacturer of Active Pharmaceutical Ingredients (APIs) and intermediates, with its manufacturing facility located in Tarapur, Maharashtra (India). The site has been su...

About the Company : Driven by passion, our pharmaceutical products, expertise and technologies accelerate our customers’ small molecule therapeutics into markets across the world. With over 35 years...

About the Company : Centaur accepts change as a constant, and continuously innovates to remain significant. Centaur has built knowledge sharing relationships with the pharmaceutical majors in areas of...

About the Company : In the dinamic pharmaceutical field, DEAFARMA is the reference point for primaries Pharmaceutical Laboratories for over twenty years, even in the national and international territo...

About the Company : Harman Finochem Limited is a leading India-based Pharmaceutical Company which specializes in the manufacture and export of more than 45 Active Pharmaceutical Ingredients (APls) of ...

About the Company : Jade Nutripharma is a global distributor of pharmaceutical, nutritional, cosmetic, animal nutrition, and agrochemical ingredients and equipment. Leveraging over 15 years of experie...

About the Company : Micro Labs Limited is a diversified healthcare company with cutting-edge R&D, advanced manufacturing facilities, and a strong distribution network. It ranks among India's top pharm...

About the Company : Established in 1984, R L Fine Chem Pvt. Ltd. is one of the fastest growing API companies, with a leadership position in several APIs such as antihistamines, antidepressants and mus...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2039-09-05
US Patent Number : 12403090
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 210833
Patent Use Code : U-724
Delist Requested :
Patent Use Description : METHOD OF TREATING SEI...
Patent Expiration Date : 2039-09-05

Patent Expiration Date : 2039-09-05
US Patent Number : 12403090
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 210833
Patent Use Code : U-724
Delist Requested :
Patent Use Description : METHOD OF TREATING SEI...
Patent Expiration Date : 2039-09-05

Patent Expiration Date : 2039-09-05
US Patent Number : 12290597
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 210833
Patent Use Code : U-724
Delist Requested :
Patent Use Description : METHOD OF TREATING SEI...
Patent Expiration Date : 2039-09-05

Patent Expiration Date : 2039-09-05
US Patent Number : 12290597
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 210833
Patent Use Code : U-724
Delist Requested :
Patent Use Description : METHOD OF TREATING SEI...
Patent Expiration Date : 2039-09-05

Patent Expiration Date : 2040-01-31
US Patent Number : 11541002
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 210833
Patent Use Code : U-724
Delist Requested :
Patent Use Description : METHOD OF TREATING SEI...
Patent Expiration Date : 2040-01-31

Patent Expiration Date : 2040-01-31
US Patent Number : 11541002
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 210833
Patent Use Code : U-724
Delist Requested :
Patent Use Description : METHOD OF TREATING SEI...
Patent Expiration Date : 2040-01-31

Patent Expiration Date : 2040-01-31
US Patent Number : 11541002
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 210833
Patent Use Code : U-724
Delist Requested :
Patent Use Description : METHOD OF TREATING SEI...
Patent Expiration Date : 2040-01-31

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
23
PharmaCompass offers a list of Clobazam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Clobazam manufacturer or Clobazam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Clobazam manufacturer or Clobazam supplier.
PharmaCompass also assists you with knowing the Clobazam API Price utilized in the formulation of products. Clobazam API Price is not always fixed or binding as the Clobazam Price is obtained through a variety of data sources. The Clobazam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Onfi manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Onfi, including repackagers and relabelers. The FDA regulates Onfi manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Onfi API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Onfi manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Onfi supplier is an individual or a company that provides Onfi active pharmaceutical ingredient (API) or Onfi finished formulations upon request. The Onfi suppliers may include Onfi API manufacturers, exporters, distributors and traders.
click here to find a list of Onfi suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Onfi DMF (Drug Master File) is a document detailing the whole manufacturing process of Onfi active pharmaceutical ingredient (API) in detail. Different forms of Onfi DMFs exist exist since differing nations have different regulations, such as Onfi USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Onfi DMF submitted to regulatory agencies in the US is known as a USDMF. Onfi USDMF includes data on Onfi's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Onfi USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Onfi suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Onfi Drug Master File in Japan (Onfi JDMF) empowers Onfi API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Onfi JDMF during the approval evaluation for pharmaceutical products. At the time of Onfi JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Onfi suppliers with JDMF on PharmaCompass.
A Onfi CEP of the European Pharmacopoeia monograph is often referred to as a Onfi Certificate of Suitability (COS). The purpose of a Onfi CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Onfi EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Onfi to their clients by showing that a Onfi CEP has been issued for it. The manufacturer submits a Onfi CEP (COS) as part of the market authorization procedure, and it takes on the role of a Onfi CEP holder for the record. Additionally, the data presented in the Onfi CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Onfi DMF.
A Onfi CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Onfi CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Onfi suppliers with CEP (COS) on PharmaCompass.
A Onfi written confirmation (Onfi WC) is an official document issued by a regulatory agency to a Onfi manufacturer, verifying that the manufacturing facility of a Onfi active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Onfi APIs or Onfi finished pharmaceutical products to another nation, regulatory agencies frequently require a Onfi WC (written confirmation) as part of the regulatory process.
click here to find a list of Onfi suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Onfi as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Onfi API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Onfi as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Onfi and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Onfi NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Onfi suppliers with NDC on PharmaCompass.
Onfi Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Onfi GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Onfi GMP manufacturer or Onfi GMP API supplier for your needs.
A Onfi CoA (Certificate of Analysis) is a formal document that attests to Onfi's compliance with Onfi specifications and serves as a tool for batch-level quality control.
Onfi CoA mostly includes findings from lab analyses of a specific batch. For each Onfi CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Onfi may be tested according to a variety of international standards, such as European Pharmacopoeia (Onfi EP), Onfi JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Onfi USP).

