



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
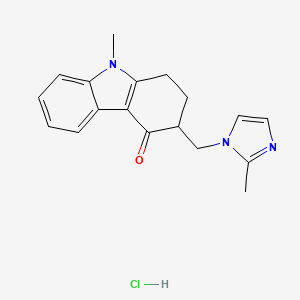

1. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-
2. Dihydrate, Ondansetron Monohydrochloride
3. Gr 38032f
4. Gr-38032f
5. Gr38032f
6. Hydrochloride, Ondansetron
7. Monohydrochloride Dihydrate, Ondansetron
8. Monohydrochloride, Ondansetron
9. Odt, Zofran
10. Ondansetron
11. Ondansetron Monohydrochloride
12. Ondansetron Monohydrochloride Dihydrate
13. Ondansetron, (+,-)-isomer
14. Ondansetron, (r)-isomer
15. Ondansetron, (s)-isomer
16. Sn 307
17. Sn-307
18. Sn307
19. Zofran
20. Zofran Odt
1. Ondansetron Hcl
2. 99614-01-4
3. Zofran
4. Ondemet
5. Emeset
6. Ondansetron (hydrochloride)
7. Zofran Odt
8. Nsc 665799
9. Ondansetronhydrochloride
10. 2999f27mad
11. Nsc-665799
12. C18h20cln3o
13. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-, Monohydrochloride
14. Emetron
15. 1,2,3,9-tetrahydro-9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-4h-carbazol-4-one Hydrochloride
16. 9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-1,2,3,9-tetrahydro-4h-carbazol-4-one Hydrochloride
17. 9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-2,3-dihydro-1h-carbazol-4(9h)-one Hydrochloride
18. Smr000469179
19. Gr 38032
20. Unii-2999f27mad
21. Sr-01000763250
22. Zofran Preservative Free
23. Zofran And Dextrose In Plastic Container
24. Cpd000469179
25. Ondansetron Hydrochloride Preservative Free
26. 9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-4h-carbazol-4-one Monohydrochloride
27. Schembl41455
28. Mls001304076
29. Mls001401397
30. Mls002222312
31. Regid_for_cid_68647
32. Gr 38032 Hcl
33. Ondansetron Hydrochloride, 98%
34. Chembl1201111
35. Ondansetron Hydrochloride- Bio-x
36. Ondansetron Hydrochloride And Dextrose In Plastic Container
37. Dtxsid701027913
38. Hms1571c18
39. Ondansetron Hydrochloride (zofran)
40. Hy-b0002
41. Ondansetron Hydrochloride And Sodium Chloride In Plastic Container
42. Mfcd00764297
43. Nsc665799
44. S1390
45. Ondansetron Hydrochloride [mi]
46. Akos015889292
47. Ab07046
48. Ccg-100852
49. Ccg-221058
50. Cs-1715
51. Gs-3597
52. H39o049
53. Nc00102
54. Ac-12464
55. Bo164177
56. Ondansetron Hydrochloride [who-dd]
57. Ondansetron Hydrochloride Anhydrous
58. O0407
59. Sw100810-5
60. Vu0424014-2
61. C90628
62. (+/-)-ondansetron Hydrochloride Anhydrous
63. A800774
64. Ondansetron Hydrochloride Anhydrous, (+/-)-
65. Q-201515
66. Sr-01000763250-5
67. Q27254405
68. 9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1h-carbazol-4-one;hydrochloride
69. 1,2,3,4-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-9h-carbazol-4-one Hydrochloride
70. 4h-carbazol-4-one, 1,2,3,9-tetrahydro-9-methyl-3-((2-methyl-1h-imidazol-1-yl)methyl)-, Hydrochloride (1:1)
71. 9-methyl-3-[(2-methyl-1h-imidazol-1-yl)methyl]-2,3,4,9-tetrahydro-1h-carbazol-4-one Hydrochloride
| Molecular Weight | 329.8 g/mol |
|---|---|
| Molecular Formula | C18H20ClN3O |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 329.1294900 g/mol |
| Monoisotopic Mass | 329.1294900 g/mol |
| Topological Polar Surface Area | 39.8 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Ondansetron hydrochloride |
| Drug Label | The active ingredient in ZOFRAN Tablets and ZOFRAN Oral Solution is ondansetron hydrochloride (HCl) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (1, 2, 3, 9-te... |
| Active Ingredient | Ondansetron hydrochloride |
| Dosage Form | Tablet; Injectable; Solution |
| Route | oral; Injection; Oral |
| Strength | eq 4mg base; eq 2mg base/ml; eq 4mg base/5ml; eq 16mg; eq 16mg base; 16mg; eq 24mg base; eq 8mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Amneal Pharms; Wockhardt; Silarx; Bedford; Hospira; Gland Pharma; Sun Pharm Inds (in); Teva; Apotex; Hikma Farmaceutica; Aurobindo Pharma; Natco Pharma; Taro; Sandoz; Par Pharm; Roxane; Glenmark Generics; Emcure Pharms; Fresenius Kabi Usa; Ipca Labs; Hikm |
| 2 of 6 | |
|---|---|
| Drug Name | Ondansetron hydrochloride preservative free |
| Active Ingredient | Ondansetron hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2mg base/ml |
| Market Status | Prescription |
| Company | Wockhardt; Hospira; Teva; Hikma Farmaceutica; Aurobindo Pharma; Sun Pharm Inds; Sandoz; Emcure Pharms; Fresenius Kabi Usa; Hikma Maple; Claris Lifesciences; Taro Pharms Ireland; Luitpold; Bedford Labs; Agila Speclts; Bd Rx |
| 3 of 6 | |
|---|---|
| Drug Name | Zofran preservative free |
| Active Ingredient | Ondansetron hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2mg base/ml |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 4 of 6 | |
|---|---|
| Drug Name | Ondansetron hydrochloride |
| Drug Label | The active ingredient in ZOFRAN Tablets and ZOFRAN Oral Solution is ondansetron hydrochloride (HCl) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (1, 2, 3, 9-te... |
| Active Ingredient | Ondansetron hydrochloride |
| Dosage Form | Tablet; Injectable; Solution |
| Route | oral; Injection; Oral |
| Strength | eq 4mg base; eq 2mg base/ml; eq 4mg base/5ml; eq 16mg; eq 16mg base; 16mg; eq 24mg base; eq 8mg base |
| Market Status | Tentative Approval; Prescription |
| Company | Amneal Pharms; Wockhardt; Silarx; Bedford; Hospira; Gland Pharma; Sun Pharm Inds (in); Teva; Apotex; Hikma Farmaceutica; Aurobindo Pharma; Natco Pharma; Taro; Sandoz; Par Pharm; Roxane; Glenmark Generics; Emcure Pharms; Fresenius Kabi Usa; Ipca Labs; Hikm |
| 5 of 6 | |
|---|---|
| Drug Name | Ondansetron hydrochloride preservative free |
| Active Ingredient | Ondansetron hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2mg base/ml |
| Market Status | Prescription |
| Company | Wockhardt; Hospira; Teva; Hikma Farmaceutica; Aurobindo Pharma; Sun Pharm Inds; Sandoz; Emcure Pharms; Fresenius Kabi Usa; Hikma Maple; Claris Lifesciences; Taro Pharms Ireland; Luitpold; Bedford Labs; Agila Speclts; Bd Rx |
| 6 of 6 | |
|---|---|
| Drug Name | Zofran preservative free |
| Active Ingredient | Ondansetron hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 2mg base/ml |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Treatment of alcohol use disorder
Serotonin 5-HT3 Receptor Antagonists
Drugs that bind to but do not activate SEROTONIN 5-HT3 RECEPTORS, thereby blocking the actions of SEROTONIN or SEROTONIN 5-HT3 RECEPTOR AGONISTS. (See all compounds classified as Serotonin 5-HT3 Receptor Antagonists.)
Antipruritics
Agents, usually topical, that relieve itching (pruritus). (See all compounds classified as Antipruritics.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)








