Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
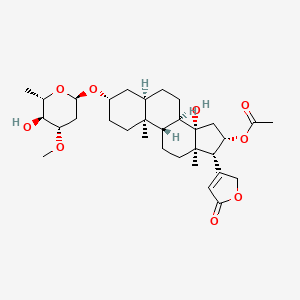

1. Foliandrin
2. Folinerin
3. Neriolin
4. Neriostene
5. 465-16-7
6. Corrigen
7. Anvirzel
8. Oleandrina
9. Oleandrine
10. Nsc-692219
11. Ii95udu7i4
12. Neriol
13. [(3s,5r,8r,9s,10s,13r,14s,16s,17r)-14-hydroxy-3-[(2r,4s,5s,6s)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-10,13-dimethyl-17-(5-oxo-2h-furan-3-yl)-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-16-yl] Acetate
14. [(3s,8r,9s,10s,13r,14s,16s,17r)-14-hydroxy-3-[(4s,5s,6s)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-10,13-dimethyl-17-(5-oxo-2h-furan-3-yl)-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-16-yl] Acetate
15. (3beta,5beta,16beta)-16-acetoxy-3-[(2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)oxy]-14-hydroxycard-20(22)-enolide
16. Oleandrina [spanish]
17. Unii-ii95udu7i4
18. Hsdb 3518
19. Einecs 207-361-5
20. Nsc 692219
21. Oleandrin [mi]
22. Oleandrigenin Oleandroside
23. Oleandrin [who-dd]
24. Schembl25049
25. Oleandrin (pbi-05204)
26. Chembl4285883
27. Oleandrin, >=98% (hplc)
28. Chebi:59030
29. Zinc8214621
30. Bdbm50465465
31. Pbi 05204
32. Pbi-05204
33. S6918
34. Stl565781
35. Akos032430539
36. Cs-5505
37. Db12843
38. 16beta-(acetyloxy)-3beta-((2,6-dideoxy-3-o-methyl-l-arabino-hexopyranosyl)oxy)-14-hydroxycard-20-(22)-enolide
39. 16beta-acetoxy-3beta-(2,6-dideoxy-3-o-methyl-l-arabino-hexopyranosyloxy)-14-hydroxycard-20(22)-enolide
40. Card-20(22)-enolide, 16-(acetyloxy)-3-((2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)oxy)-14-hydroxy-, (3beta,5beta,16beta)-
41. Hy-13719
42. N2088
43. C19987
44. Q411532
45. (3beta,16beta)-16-(acetyloxy)-3-((2,6-dideoxy-3-o-methyl-l-arabino-hexopyranosyl)oxy)-14-hydroxy Card-20(22)-enolide
46. (3beta,5beta,16beta)-16-(acetyloxy)-3-[(2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)oxy]-14-hydroxycard-20(22)-enolide
47. 1315607-79-4
48. Card-20(22)-enolide, 16-(acetyloxy)-3-((2,6-dideoxy-3-o-methyl-.alpha.-l-arabino-hexopyranosyl)oxy)-14-hydroxy-, (3.beta.,5.beta.,16.beta.)-
49. Card-20(22)-enolide, 16-(acetyloxy)-3-((2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)oxy)-14-hydroxy-, (3-beta,5-beta,16-beta)-
| Molecular Weight | 576.7 g/mol |
|---|---|
| Molecular Formula | C32H48O9 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 576.32983310 g/mol |
| Monoisotopic Mass | 576.32983310 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 1080 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 13 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
The purpose of this study was to examine the mechanism(s) and differential cell-killing effects of Anvirzel, an extract of oleander (Nerium oleander; family-Apocynaceae), and its derivative compound Oleandrin on human, canine and murine tumor cells. Cells received different concentrations of Anvirzel (1.0 ng/mL to 500 ug/mL) or Oleandrin (0.01 ng/ml to 50 ug/mL) in both continuously treated and pulse-treated/recovery cultures. The cytotoxicity of these compounds was then determined. Both Anvirzel and Oleandrin were able to induce cell killing in human cancer cells, but not in murine cancer cells; the cell-killing potency of Oleandrin was greater than that of Anvirzel. Canine oral cancer cells treated with Anvirzel showed intermediate levels of response, with some abnormal metaphases and cell death resulting from the treatment. From these results we conclude that Anvirzel and Oleandrin act in a species-specific manner, and while testing the effectiveness of a new compound for cancer treatment, one must use not only murine but a variety of cancer cells, including those of human origin.
PMID:11001386 Pathak S et al; Anticancer Drugs 11 (6): 455-63 (2000)
/EXPL THER/ Oleandrin, derived from the leaves of Nerium oleander, has been shown to possess anti-inflammatory and tumor cell growth-inhibitory effects. Here, we provide evidence that oleandrin could possess anti-tumor promoting effects. We determined the effect of topical application of oleandrin to CD-1 mice against l2-O-tetradecanoylphorbol-13-acetate (TPA), a widely studied skin tumor promoter, -induced conventional and novel markers of skin tumor promotion. Topical application of oleandrin (2 mg per mouse) 30 min before TPA (3.2 nmol per mouse) application onto the skin afforded significant inhibition, in a time-dependent manner, against TPA-mediated increase in cutaneous edema and hyperplasia, epidermal ornithine decarboxylase (ODC) activity and ODC and cyclooxgenase-2 (COX-2) protein expression. In search for novel markers of skin tumor promotion, we found that TPA application to mouse skin resulted, as an early event, in an increased expression of phosphatidyinositol 3-kinase (PI3K), phosphorylation of Akt at threonine308 and activation of nuclear factor kappa B (NF-kappaB). Topical application of oleandrin before TPA application to mouse skin resulted in significant reduction in TPA-induced expression of PI3K and phosphorylation of Akt, and inhibition of NF-kappaB activation. NF-kappaB is a eukaryotic transcription factor that is critically involved in regulating the expression of specific genes that participate in inflammation, apoptosis and cell proliferation. Employing Western blot analysis, we found that oleandrin application to mouse skin resulted in inhibition of TPA-induced activation of NF-kappaB, IKKalpha and phosphorylation and degradation of IkappaBalpha. Our data suggest that oleandrin could be a useful anti-tumor promoting agent because it inhibits several biomarkers of TPA-induced tumor promotion in an in vivo animal model. One might envision the use of chemopreventive agents such as oleandrin in an emollient or patch for chemoprevention or treatment of skin cancer.
PMID:15020199 Afaq F et al; Toxicol Appl Pharmacol 195 (3): 361-9 (2004)
/EXPL THER/ NF-kappaB is a ubiquitous and well-characterized protein responsible for the regulation of complex phenomena, with a pivotal role in controlling cell signaling in the body under certain physiological and pathological conditions. Among other functions, NF-kappaB controls the expression of genes encoding the pro-inflammatory cytokines (e. g., IL-1, IL-2, IL-6, TNF-alpha, etc.), chemokines (e. g., IL-8, MIP-1alpha, MCP1, RANTES, eotaxin, etc.), adhesion molecules (e. g., ICAM, VCAM, E-selectin), inducible enzymes (COX-2 and iNOS), growth factors, some of the acute phase proteins, and immune receptors, all of which play critical roles in controlling most inflammatory processes. Since NF-kappaB represents an important and very attractive therapeutic target for drugs to treat many inflammatory diseases, including arthritis, asthma, and the auto-immune diseases, most attention has been paid in the last decade to the identification of compounds that selectively interfere with this pathway. Recently, a great number of plant-derived substances have been evaluated as possible inhibitors of the NF-kappaB pathway. These include a wide range of compound classes, such as lignans (manassantins, (+)-saucernetin, (-)-saucerneol methyl ether), sesquiterpenes (costunolide, parthenolide, celastrol, celaphanol A), diterpenes (excisanin, kamebakaurin), triterpenes (avicin, oleandrin), polyphenols (resveratrol, epigallocatechin gallate, quercetin), etc. In this mini-review we will discuss the medicinal chemistry of these compounds with regards to the NF-kappaB inhibition.
PMID:16918500 Nam NH et al; Mini Rev Med Chem 6 (8): 945-51 (2006)
/EXPL THER/ The treatment of cancer with chemotherapeutic agents and radiation has two major problems: time-dependent development of tumor resistance to therapy (chemoresistance and radioresistance) and nonspecific toxicity toward normal cells. Many plant-derived polyphenols have been studied intently for their potential chemopreventive properties and are pharmacologically safe. These compounds include genistein, curcumin, resveratrol, silymarin, caffeic acid phenethyl ester, flavopiridol, emodin, green tea polyphenols, piperine, oleandrin, ursolic acid, and betulinic acid. Recent research has suggested that these plant polyphenols might be used to sensitize tumor cells to chemotherapeutic agents and radiation therapy by inhibiting pathways that lead to treatment resistance. These agents have also been found to be protective from therapy-associated toxicities. How these polyphenols protect normal cells and sensitize tumor cells to treatment is discussed in this review.
PMID:16356126 Garg AK et al; Antioxid Redox Signal 7 (11-12): 1630-47 (2005)
/EXPL THER/ The principal active constituent of the botanical drug candidate PBI-05204, a supercritical CO(2) extract of Nerium oleander, is the cardiac glycoside oleandrin. PBI-05204 shows potent anticancer activity and is currently in phase I clinical trial as a treatment for patients with solid tumors. We have previously shown that neriifolin, which is structurally related to oleandrin, provides robust neuroprotection in brain slice and whole animal models of ischemic injury. However, neriifolin itself is not a suitable drug development candidate and the FDA-approved cardiac glycoside digoxin does not cross the blood-brain barrier. We report here that both oleandrin as well as the full PBI-05204 extract can also provide significant neuroprotection to neural tissues damaged by oxygen and glucose deprivation as occurs in ischemic stroke. Critically, we show that the neuroprotective activity of PBI-05204 is maintained for several hours of delay of administration after oxygen and glucose deprivation treatment. We provide evidence that the neuroprotective activity of PBI-05204 is mediated through oleandrin and/or other cardiac glycoside constituents, but that additional, non-cardiac glycoside components of PBI-05204 may also contribute to the observed neuroprotective activity. Finally, we show directly that both oleandrin and the protective activity of PBI-05204 are blood brain barrier penetrant in a novel model for in vivo neuroprotection. Together, these findings suggest clinical potential for PBI-05204 in the treatment of ischemic stroke and prevention of associated neuronal death.
PMID:21950737 Dunn DE et al; J Neurochem 119 (4): 805-14 (2011)
Pharmacokinetic studies of (3)H oleandrin, a cardiac glycoside component of Anvirzel, were conducted in mice after either an i.v. dose (40 ug/kg) or a p.o. dose (80 ug/kg). Oleandrin was rapidly absorbed after oral dosing (Cmax at 20 min) although the elimination half-life was longer (2.3 +/- 0.5 hr) than that after i.v. dosing (0.4 +/- 0.1 hr). The AUC0-infinity values obtained after i.v. and p.o. dosing were 24.6 +/- 11.1 and 14.4 +/- 4.3 (ng.hr/mL), respectively, resulting in an oral bioavailability of approximately 30%. After i.v. administration, oleandrin concentration in liver was approximately twice that measured in heart or kidney tissue. Oleandrigenin, the aglycone of oleandrin, was also found in these tissues. At 5 min, > 60% of the total radioactivity in liver was due to oleandrin while 28% of the given dose was present as oleandrigenin. Twenty-four hours following injection, 8% of total radioactivity was excreted in urine and contained both oleandrigenin (4.4% of the injected dose) and oleandrin (1.9%). Sixty-six percent of injected radioactivity was found in feces and consisted of oleandrin and oleandrigenin in equal amounts. Uptake of oleandrin in brain after i.p. injection of oleandrin (3 mg/kg) or oleander extract (700 mg/kg) was examined. Measured by LC/MS/MS, oleandrin content in brain was higher following injection of extract than it was with an equivalent dose of oleandrin. The data suggest that components within oleander extract may enhance transport of oleandrin across the blood brain barrier.
PMID:12416031 Ni D et al; J Exp Ther Oncol 2 (5): 278-85 (2002)
The toxicity due to an infusion or decoction of N oleander into rabbits was attributed to the oleandrin content in various organs. The heart, stomach, kidneys, and blood contained the greatest oleandrin concentrations, whereas the lung and brain contained none.
Bors G et al; Toxicology of Nerium oleander; Pharmazie 26 (12) 764 (1971)
Agents that can suppress the activation of nuclear factor-kappaB (NF-kappaB) and activator protein-1 (AP-1) may be able to block tumorigenesis and inflammation. Oleandrin, a polyphenolic cardiac glycoside derived from the leaves of Nerium oleander, is a candidate NF-kappaB and AP-1 modulator. We investigated the effect of oleandrin on NF-kappaB activation induced by inflammatory agents. Oleandrin blocked tumor necrosis factor (TNF)-induced activation of NF-kappaB in a concentration- and time-dependent manner. This effect was mediated through inhibition of phosphorylation and degradation of IkappaBalpha, an inhibitor of NF-kappaB. A proprietary hot water extract of oleander (Anvirzel) also blocked TNF-induced NF-kappaB activation; subsequent fractionation of the extract revealed that this activity was attributable to oleandrin. The effects of oleandrin were not cell type specific, because it blocked TNF-induced NF-kappaB activation in a variety of cells. NF-kappaB-dependent reporter gene transcription activated by TNF was also suppressed by oleandrin. The TNF-induced NF-kappaB activation cascade involving TNF receptor 1/TNF receptor-associated death domain/TNF receptor-associated factor 2/NF-kappaB-inducing kinase/IkappaBalpha kinase was interrupted at the TNF receptor-associated factor 2 and NF-kappaB-inducing kinase sites by oleandrin, thus suppressing NF-kappaB reporter gene expression. Oleandrin blocked NF-kappaB activation induced by phorbol ester and lipopolysaccharide. Oleandrin also blocked AP-1 activation induced by TNF and other agents and inhibited the TNF-induced activation of c-Jun NH2-terminal kinase. Overall, our results indicate that oleandrin inhibits activation of NF-kappaB and AP-1 and their associated kinases. This may provide a molecular basis for the ability of oleandrin to suppress inflammation and perhaps tumorigenesis.
PMID:10919658 Manna SK et al; Cancer Res 60 (14): 3838-47 (2000)
Cardiac glycosides are very effective to kill human cells, but not murine cells. In this report, we describe the comparative molecular mechanism of oleandrin, a cardiac glycoside action in human and murine cells. Treatment with oleandrin facilitated nuclear translocation of FKHR in human, but not murine cells by dephosphorylating Akt. It activated MAPK and JNK in human, but not in murine cells and also induced expression of FasL leads to apoptosis in human cells as detected by assaying caspases activation, PARP cleavage, nuclear fragmentation, and annexin staining. Oleandrin interacted with human plasma membrane as evaluated by HPLC, altered its fluidity as detected by DPH binding, inhibited Na+/K+-ATPase activity, and increased intracellular free Ca2+ level followed by calcineurin activity only in human, but not in murine cells. Results suggest that human plasma membrane might be different than murine, which interact with oleandrin that disturb Na+/K+-ATPase pump resulting in the calcification followed by induction of Ca2+-dependent cellular responses such as apoptosis.
PMID:17173971 Raghavendra PB et al; Mol Immunol 44 (9): 2292-302 (2007)
ABOUT THIS PAGE
70
PharmaCompass offers a list of Oleandrin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Oleandrin manufacturer or Oleandrin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Oleandrin manufacturer or Oleandrin supplier.
PharmaCompass also assists you with knowing the Oleandrin API Price utilized in the formulation of products. Oleandrin API Price is not always fixed or binding as the Oleandrin Price is obtained through a variety of data sources. The Oleandrin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Oleandrin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Oleandrin, including repackagers and relabelers. The FDA regulates Oleandrin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Oleandrin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Oleandrin supplier is an individual or a company that provides Oleandrin active pharmaceutical ingredient (API) or Oleandrin finished formulations upon request. The Oleandrin suppliers may include Oleandrin API manufacturers, exporters, distributors and traders.
Oleandrin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Oleandrin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Oleandrin GMP manufacturer or Oleandrin GMP API supplier for your needs.
A Oleandrin CoA (Certificate of Analysis) is a formal document that attests to Oleandrin's compliance with Oleandrin specifications and serves as a tool for batch-level quality control.
Oleandrin CoA mostly includes findings from lab analyses of a specific batch. For each Oleandrin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Oleandrin may be tested according to a variety of international standards, such as European Pharmacopoeia (Oleandrin EP), Oleandrin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Oleandrin USP).

