



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers

USA (Orange Book)
0

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
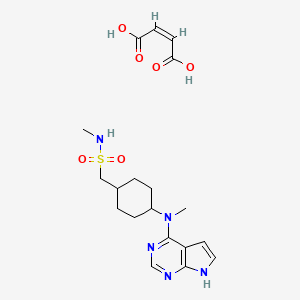

1. Apoquel
2. Oclacitinib
1. 1208319-27-0
2. Oclacitinib (maleate)
3. 1640292-55-2
4. Apoquel
5. Oclacitinib Maleate [usan]
6. Oclacitinib Maleate(pf-03394197)
7. Pf-03394197-11
8. Von733l42a
9. Oclacitinib Maleate (usan)
10. (z)-but-2-enedioic Acid;n-methyl-1-[4-[methyl(7h-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclohexyl]methanesulfonamide
11. N-methyl(trans-4-(methyl-7h-pyrrolo(2,3-d)pyrimidin-4-ylamino)cyclohexyl) Methanesulfonamide (2z)-2-butenedioate
12. Unii-von733l42a
13. Oclacitinib(maleate)
14. Pf-03394197 Maleate
15. Schembl260868
16. Chembl2105739
17. Oclacitinib Maleate [mi]
18. Schembl19205843
19. Amy19492
20. Ex-a3658
21. Hy-13577a
22. S8195
23. Ccg-269269
24. Cs-7611
25. Ac-32980
26. Bs-44681
27. D10142
28. D84061
29. Oclacitinib Maleate (ema Epar: Veterinary)
30. Pf 03394197-11
31. Q27291934
32. Cyclohexanemethanesulfonamide, N-methyl-4-(methyl-7h-pyrrolo(2,3-d)pyrimidin-4- Ylamino)-, Trans-, (2z)-2-butenedioate (1:?)
33. N-methyl-1-((1r,4r)-4-(methyl(7h-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclohexyl)methanesulfonamide Maleate
34. N-methyl-1-(trans-4-(methyl(7h-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclohexyl)methanesulfonamide Maleate(1:x)
| Molecular Weight | 453.5 g/mol |
|---|---|
| Molecular Formula | C19H27N5O6S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 453.16820477 g/mol |
| Monoisotopic Mass | 453.16820477 g/mol |
| Topological Polar Surface Area | 174 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 606 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
- Treatment of pruritus associated with allergic dermatitis in dogs.
- Treatment of clinical manifestations of atopic dermatitis in dogs.
QD11AH90


