



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
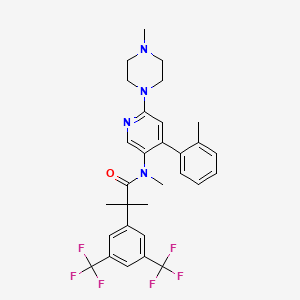

1. 2-(3,5-bis(trifluoromethyl)phenyl)-n,2-dimethyl-n-(4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl)propanamide
2. 2-(3,5-bis(trifluoromethyl)phenyl)-n-methyl-n-(4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl)-2-methylpropanamide
3. Benzeneacetamide, N, Alpha, Alpha-trimethyl-n-(4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl)-3,5-bis(trifluoromethyl)-
4. Benzeneacetamide, N, Alpha,alpha-trimethyl-n-(4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl)-3,5-bis(trifluoromethyl)-, Hydrochloride (1:2)
5. Netupitant Dihydrochloride
6. Netupitant Hydrochloride
7. Ro 67-3189 000
8. Ro 673189000
9. Ro-67-3189
10. Ro-67-3189 000
11. Ro-673189000
1. 290297-26-6
2. 2-[3,5-bis(trifluoromethyl)phenyl]-n,2-dimethyl-n-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
3. 2-(3,5-bis(trifluoromethyl)phenyl)-n,2-dimethyl-n-(6-(4-methylpiperazin-1-yl)-4-(o-tolyl)pyridin-3-yl)propanamide
4. Ro-67-3189
5. Ro-673189000
6. Ro 67-3189/000
7. Chembl206253
8. Chebi:85155
9. 7732p08tir
10. Cid 6451149
11. Ro 67-31898/000
12. Benzeneacetamide, N,alpha,alpha-trimethyl-n-[4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl]-3,5-bis(trifluoromethyl)-
13. 2-(3,5-bis(trifluoromethyl)phenyl)-n,2-dimethyl-n-(4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl)propanamide
14. 2-(3,5-bis(trifluoromethyl)phenyl)-n,2-dimethyl-n-(6-(4-methylpiperazin-1-yl)-4-o-tolylpyridin-3-yl)propanamide
15. 2-[3,5-bis(trifluoromethyl)phenyl]-~{n},2-dimethyl-~{n}-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
16. Netupitant [usan]
17. Netupitant [usan:inn]
18. Unii-7732p08tir
19. Benzeneacetamide, N,alpha,alpha-trimethyl-n-(4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl)-3,5-bis(trifluoromethyl)-
20. Cid6451149
21. Netupitant [mi]
22. Netupitant [inn]
23. Netupitant (usan/inn)
24. Netupitant [vandf]
25. Netupitant [who-dd]
26. Schembl445804
27. Gtpl5742
28. Netupitant(cid-6451149)
29. Netupitant [orange Book]
30. Dtxsid50183271
31. Glxc-15009
32. Hms3887e19
33. Bcp08751
34. Ex-a2747
35. Bdbm50178574
36. Mfcd25976831
37. S4654
38. Zinc11681563
39. Akos027251056
40. Ccg-270121
41. Cs-3337
42. Db09048
43. Sb18973
44. Cid-6451149
45. Ncgc00390569-01
46. 2-(3,5-bis(trifluoromethyl)phenyl)-n-methyl-n-(4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl)-2-methylpropanamide
47. Ac-29232
48. As-10239
49. Hy-16346
50. Ro 67-31898
51. Akynzeo Capsule Component Netupitant
52. Netupitant Component Akynzeo Capsule
53. Ft-0699740
54. A14428
55. D05152
56. Ro-67-3189/000
57. Q19598139
58. 2-(3,5-bis(trifluoromethyl)phenyl)-n,2-dimethyl-n-(6-(4-methylpiperazin-1-yl)-4-o-tolylpyridin-3-yl)propanamide, Aldrichcpr
59. 2-(3,5-bis(trifluoromethyl)phenyl)-n,2-dimethyl-n-(6-(4-methylpiperazin-1yl)-4-(o-tolyl)pyridin-3yl)propanamide
60. 2-(3,5-bis-trifluoromethyl-phenyl)-n-methyl-n-[6-(4-methyl-piperazin-1-yl)-4-o-tolyl-pyridin-3-yl]-isobutyramide
61. 2-[3,5-bis(trifluoromethyl)phenyl]-n,2-dimethyl-n-[4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl]propanamide
62. Benzeneacetamide, N,.alpha.,.alpha.-trimethyl-n-(4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl)-3,5-bis(trifluoromethyl)-
63. Gaw
64. N,alpha,alpha-trimethyl-n-[4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl]-3,5-bis(trifluoromethyl)-benzeneacetamide
| Molecular Weight | 578.6 g/mol |
|---|---|
| Molecular Formula | C30H32F6N4O |
| XLogP3 | 6.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 5 |
| Exact Mass | 578.24803063 g/mol |
| Monoisotopic Mass | 578.24803063 g/mol |
| Topological Polar Surface Area | 39.7 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 865 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Netupitant is an antiemitic drug approved by the FDA in October 2014 for use in combination with palonosetron for the prevention of acute and delayed vomiting and nausea associated with cancer chemotherapy including highly emetogenic chemotherapy.
FDA Label
Absorption
Upon oral administration of a single dose of netupitant, netupitant started to be measurable in plasma between 15 minutes and 3 hours after dosing. Plasma concentrations reached Cmax in approximately 5 hours. There was a greater than dose-proportional increase in the systemic exposure with the dose increase from 10 mg to 300 mg and a dose-proportional increase in systemic exposure with a dose increase from 300 mg to 450 mg.
Route of Elimination
Primarily fecal.
Volume of Distribution
In cancer patients, Vz/F: 1982 906 L (mean SD).
Clearance
Estimated systemic clearance of 20.3 9.2 L/h (mean SD).
Once absorbed, netupitant is extensively metabolized to form three major metabolites: desmethyl derivative, M1; N-oxide derivative, M2; and OH-methyl derivative, M3. Metabolism is mediated primarily by CYP3A4 and to a lesser extent by CYP2C9 and CYP2D6. Metabolites M1, M2 and M3 were shown to bind to the substance P/neurokinin 1 (NK1) receptor.
96 hours with CV% of 61.
Delayed emesis (vomiting) has been largely associated with the activation of tachykinin family neurokinin 1 (NK1) receptors (broadly distributed in the central and peripheral nervous systems) by substance P. As shown in in vitro and in vivo studies, netupitant inhibits substance P mediated responses.






