



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
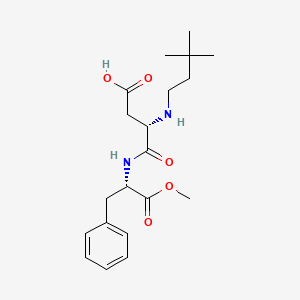

1. N-(n-(3,3-dimethylbutyl)-l-alpha-aspartyl)-l-phenylalanine 1-methyl Ester
2. Nc 00723
1. 165450-17-9
2. (s)-3-((3,3-dimethylbutyl)amino)-4-(((s)-1-methoxy-1-oxo-3-phenylpropan-2-yl)amino)-4-oxobutanoic Acid
3. Vj597d52ex
4. Nc 00723
5. Hsdb 7965
6. Nc-00723
7. (s)-3-((3,3-dimethylbutyl)amino)-4-(((s)-1-methoxy-1-oxo-3-phenylpropan-2-yl)amino)-4-oxobutanoic Ac
8. Neotame [nf]
9. L-phenylalanine, N-(3,3-dimethylbutyl)-l-alpha-aspartyl-, 2-methyl Ester
10. (3s)-3-(3,3-dimethylbutylamino)-4-[[(2s)-1-methoxy-1-oxo-3-phenylpropan-2-yl]amino]-4-oxobutanoic Acid
11. N-(n-(3,3-dimethylbutyl)-l-alpha-aspartyl)-l-phenylalanine 1-methyl Ester
12. Unii-vj597d52ex
13. Mfcd09039056
14. Neotame [fhfi]
15. Neotame [inci]
16. Neotame [fcc]
17. Neotame [ii]
18. Neotame [mi]
19. Neotame [mart.]
20. Neotame [usp-rs]
21. Mirasee 200
22. Schembl4311
23. Neotame, Analytical Standard
24. Neotame (200 Mg)
25. Ins No.961
26. Chembl3718532
27. Chebi:83503
28. Dtxsid50167950
29. Ins-961
30. Neotame 1000 Microg/ml In Water
31. Methyl N-(3,3-dimethylbutyl)-l-alpha-aspartyl-l-phenylalaninate
32. S4442
33. Zinc33965935
34. Akos016842430
35. Am84564
36. Bcp9000982
37. Cs-w011769
38. Gs-3213
39. Hy-w011053
40. (3s)-3-[(3,3-dimethylbutyl)amino]-3-{[(2s)-1-methoxy-1-oxo-3-phenylpropan-2-yl]carbamoyl}propanoic Acid
41. Bcp0726000005
42. E 961
43. E-961
44. N1112
45. 450n179
46. A810640
47. Q415698
48. J-010204
49. N-[n-(3,3-dimethylbutyl)-l-alpha-aspartyl]-l-phenylalanine Methyl Ester
50. L-phenylalanine, N-(n-(3,3-dimethylbutyl)-l-.alpha.-aspartyl)-1-methyl Ester
51. N-(n-(3,3-dimethylbutyl)-l-.alpha.-aspartyl)-l-phenylalanine 1-methyl Ester
52. (3s)-4-[[(1s)-1-benzyl-2-methoxy-2-oxo-ethyl]amino]-3-(3,3-dimethylbutylamino)-4-oxo-butanoic Acid
53. (s)-3-((3,3-dimethylbutyl)amino)-4-(((s)-1-methoxy-1-oxo-3-phenylpropan-2-yl)amino)-4-oxobutanoicacid
| Molecular Weight | 378.5 g/mol |
|---|---|
| Molecular Formula | C20H30N2O5 |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 12 |
| Exact Mass | 378.21547206 g/mol |
| Monoisotopic Mass | 378.21547206 g/mol |
| Topological Polar Surface Area | 105 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 495 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
14(C)-neotame was administered to groups of six male and six female Sprague-Dawley Crl:CDBR rats by gavage or by intravenous injection as a single dose of 15 mg/kg bw. Rats were individually housed in metabolism cages and urine and feces were collected at intervals for 72 hr after dosing. A additional group of three rats received a single oral dose of 120 mg/kg bw. All rats were killed after 72 hr and the carcasses were retained for analysis. Radiolabel was measured in all samples and the metabolites present in the urine and feces were determined. After oral administration, >90% of the radiolabel was recovered in urine and faeces within 48 hr. Within 72 hr after oral administration of (14)C-neotame at a dose of 15 or 120 mg/kg bw, 8.5-10.8% and 84.5-87.2% of the radiolabel was excreted in the urine and feces, respectively. After intravenous administration of (14)C-neotame at dose of 15 mg/kg bw, approximately 35% and 59% of the radiolabel was recovered in urine and feces, respectively. Less than 0.3% of the radiolabel was recovered in the carcasses within 72 hr after either oral or intravenous administration. Unchanged neotame was only detected in urine collected from female rats 0-6 h after intravenous administration and accounted for 3.7% of the administered dose. Unchanged neotame was not detected in the feces of any animal regardless of the dose or route of administration.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 52: Neotame (165450-17-9) (2004). Available from, as of July 18, 2011: https://www.inchem.org/pages/jecfa.html
Sprague-Dawley Crl:CDBRVAF Plus rats were each given a single oral dose of 15 mg/kg bw of (14)C-neotame by gavage and divided among four groups. In rats in group 1 (three rats of each sex), blood was taken at intervals up to 24 hr after treatment, separated into cell and plasma fractions, and analysed for radiolabel. Rats in group 2 (two rats of each sex) were housed in glass metabolism cages for 72 hr after treatment for collection of urine, feces and expired air. Carcasses were solubilized for analysis of retained radiolabel, and urine and feces were pooled for analysis of metabolites as well as total radiolabel. In group 3 (two rats of each sex), rats were anesthetized 0.5 hr or 2 hr after dosing, and blood was collected and analysed. Rats in group 4 (two males) were anesthetized and the bile ducts and stomach cannulated. Radiolabelled neotame was administered via the stomach cannula, and bile was collected at intervals up to 48 hr after treatment. Urine and feces were collected for 0-24 hr and 24-48 hr and radiolabel was measured. Plasma concentrations of radiolabel after oral dosing with (14)C-neotame peaked at 30 min after dosing in females and 1 h after dosing in males, followed by a rapid decline. The major metabolite identified in plasma, urine, feces and bile was de-esterified neotame. The excretion of (14)C-neotame was examined over 72 hr; 8-10% of the radiolabel was recovered in urine, 90-92% in feces, and 0.01-0.03% in expired air. After 72 hr, 0.11-0.13% of the radiolabel remained in the carcass. In males, urinary excretion was virtually complete within 12 hr, while in females, urinary excretion continued over 24 hr. Most fecal excretion occurred between 6 hr and 24 hr after dosing in both sexes. In males in group 4, urinary excretion was similar to that seen in other groups, with around 5-9% of the administered dose being excreted in the urine. Biliary excretion accounted for approximately 5.7% of the administered dose, while fecal excretion accounted for around 85% of the administered dose. Little radiolabel was retained in the carcass.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 52: Neotame (165450-17-9) (2004). Available from, as of July 18, 2011: https://www.inchem.org/pages/jecfa.html
In a study designed to examine the distribution and elimination of radioactivity derived from neotame by whole-body autoradiography, eight pregnant and eight non-pregnant Sprague-Dawley rats were each given a single dose of 15 mg/kg bw of (14)C-neotame by gavage. The rats were sacrificed at various times up to 24 hr after dosing and the carcasses treated as in the previous study. The tissue distribution of radiolabel was similar in pregnant and non-pregnant rats. Placental concentrations of radiolabel were low at 0.5 and 2 hr after dosing, similar to those seen in other peripheral tissues and in circulating blood. No radiolabel was detected in the fetus at any time. The highest concentrations of radiolabel were seen shortly after dosing, initially in the stomach contents, gastrointestinal tract, liver, kidneys and bladder, with lower concentrations in the rest of the body. At subsequent time-points, the passage of radiolabel through the excretory organs was seen. No accumulation was seen in tissues, and concentrations were very low after 24 hr. There was no significant difference between pregnant and non-pregnant rats in the time profile with which radiolabel was distributed in the tissues
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 52: Neotame (165450-17-9) (2004). Available from, as of July 18, 2011: https://www.inchem.org/pages/jecfa.html
In a ... study to examine the distribution of neotame in rat tissues, 21 male Lister Hooded rats were given (14)C-neotame in a single oral dose of 15 mg/kg bw by gavage. Pairs of rats (one of each sex) were killed after 0.5, 2, 6, 12 and 24 hr, pinned out, frozen rapidly, and sagittal sections taken through the carcass at six levels were examined by autoradiography. Qualitative assessment of radiolabel present in male and female rats indicated that the highest levels were present in rats killed at the earliest time-points after dosing. Levels decreased rapidly with time. At 0.5 hr and 2 hr after dosing, most radiolabel was found in the stomach, the gastrointestinal tract, liver, kidneys and bladder, with smaller amounts being distributed throughout the rest of the body. Very small amounts were found in the central nervous system, and no binding to pigmented skin or the eye was observed. Levels were consistent with the circulation of radiolabel in the bloodstream. At subsequent time-points (6, 12 and 24 hr), the passage of radiolabel through the excretory organs was seen. By 24 hr after dosing, only very small amounts remained in the animal and there was no evidence of accumulation in any tissue.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 52: Neotame (165450-17-9) (2004). Available from, as of July 18, 2011: https://www.inchem.org/pages/jecfa.html
For more Absorption, Distribution and Excretion (Complete) data for Neotame (11 total), please visit the HSDB record page.
... Neotame was labelled with Carbon-14 at the 1-position in the dimethylbutyl side chain and with Carbon-13 in the 2 terminal methyl groups of the same side chain. ... Volunteers ingested a single dose of the labelled test substance in water at a level approximately equivalent to 0.25 mg/kg, which corresponds to the amount of neotame needed to sweeten 1 L of beverage. Neotame was rapidly, but incompletely absorbed and rapidly excreted. A mean of 98% of the administered radioactivity was recovered in urine and feces, mostly within 72 hours of dosing. Mean plasma concentrations of neotame peaked at 0.4 hr ... and declined with a half-life of 0.6 hr. The major metabolite of neotame was de-esterified neotame formed by hydrolysis of the methyl ester group. Mean plasma concentrations of this metabolite peaked at 1 hr ... /and/ were approximately 2.5 times higher than neotame concentrations and declined with a half-life of 1.5 hr. De-esterified neotame represented a mean of approximately 80% of the excreted dose. Two other metabolites were detected at greater than 1% of the dose. One, that was a mean of about 4.9% of the dose, was found in the feces and was identified as N-(3, 3 dimethylbutyl)-L aspartic acid. The other metabolite was in urine and was identified by LC/MS/MS, NMR and original synthesis as a carnitine ester of 3, 3-dimethylbutanoic acid. All metabolites of neotame present at 1% or greater of the dose have been shown to occur in the species used in safety studies, confirming the safety of these metabolites.
Aikens P, et al; Toxicologist 78 (1-S): 207 (2004)
... As part of the safety testing, studies were conducted to evaluate the absorption, distribution, pharmacokinetics, metabolism and excretion of neotame in laboratory rats and dogs. For this purpose, neotame was labelled with Carbon-14 at the 1-position in the dimethylbutyl side chain and was administered to animals at doses of 15 or 120 mg/kg body weight. In rats and dogs, oral doses of neotame were rapidly, but incompletely absorbed and rapidly excreted with no evidence of potential for accumulation. In rats, absorbed Carbon-14 was mainly associated with the gastrointestinal tract and organs of metabolism and excretion (liver, kidney and bladder). Almost no neotame was detected in (stabilised) plasma or .../excretions/ after oral dosing to rats. This was probably due to high activity of plasma esterases. The major metabolite of neotame was de-esterified neotame formed by hydrolysis of the methyl ester group. In rats, mean plasma concentrations of this metabolite peaked at 0.5 hr ...and declined with a half life of 1 hr. In dogs, which have a lower level of plasma esterase activity, neotame was detected in plasma and ... /excretions/ after oral dosing. Mean plasma concentrations of neotame peaked at 0.5 hr ... and declined with a half-life of 0.4 hr. Deesterified neotame represented a mean of approximately 70-80% of excreted oral doses in both rats and dogs. Other metabolites detected included N-(3, 3 dimethylbutyl)- L aspartic acid (in rats and dogs about 2% of the dose) and a beta-glucuronide conjugate of 3, 3-dimethylbutanoic acid (in rats and dogs about 5% of the dose). In addition the carnitine ester of 3, 3-dimethylbutanoic acid was present in the urine of female rats.
Mayhew D, et al; Toxicologist 78 (1-S): 208 (2004)
After oral administration, approximately 20-30% of the administered dose is absorbed and rapidly converted to the major metabolite, N-(N-(3,3-dimethylbutyl)-L-alpha-aspartyl)-L-phenylalanine (de-esterified neotame) and a number of minor metabolites. Neotame and its metabolites are rapidly eliminated in the urine and feces. ... The major metabolic pathway is de-esterification of neotame to N-[N-(3,3-dimethylbutyl)-L-alpha-aspartyl]-L-phenylalanine and methanol. Minor metabolites are N-(3,3-dimethylbutyl)-L-aspartic acid, a metabolite formed via peptide or amide hydrolysis of neotame; 3,3-dimethylbutyric acid, also referred to as 3,3-dimethylbutanoic acid; the carnitine conjugate of 3,3-dimethylbutyric acid; and the glucuronide conjugate of 3,3-dimethylbutyric acid.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 52: Neotame (165450-17-9) (2004). Available from, as of July 18, 2011: https://www.inchem.org/pages/jecfa.html
14(C)-neotame was administered to groups of six male and six female Sprague-Dawley Crl:CDBR rats by gavage or by intravenous injection as a single dose of 15 mg/kg bw. Rats were individually housed in metabolism cages and urine and feces were collected at intervals for 72 hr after dosing. A additional group of three rats received a single oral dose of 120 mg/kg bw. All rats were killed after 72 hr and the carcasses were retained for analysis. Radiolabel was measured in all samples and the metabolites present in the urine and feces were determined. ... The major metabolite found in urine after 48 hr was de-esterified neotame, independent of the route of administration or the dose. N-(3,3-dimethylbutyl)-L-aspartic acid (NC-00754) was detected at lower concentrations (around 10% of the levels of de-esterified neotame after oral dosing). Parent compound was found only in the urine of female rats after intravenous dosing (3.7% of the dose); none was detected in the urine of any other groups. A glucuronide metabolite was also detected at low levels (0.4-0.5% of the administered dose) in the urine, independent of dose or route of administration. Two minor metabolites, each representing <1.6% of the administered dose, were identified. In the feces, de-esterified neotame was the major metabolite (approximately 70-80% of the dose after oral administration). N(3,3dimethylbutyl)Laspartic acid (NC-00754) was detected at lower levels, 0.8-2.5% of the dose. Low concentrations of an unidentified metabolite were also found, representing 0.7-1.2% of the administered dose.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 52: Neotame (165450-17-9) (2004). Available from, as of July 18, 2011: https://www.inchem.org/pages/jecfa.html
For more Metabolism/Metabolites (Complete) data for Neotame (9 total), please visit the HSDB record page.
Healthy men (mean age + or - standard deviation (SD), 28 + or - 6 years) were each given a single dose of neotame in solution at 0.10, 0.25 or 0.50 mg/kg bw (n =7, 6, and 6 men per dose, respectively), after an overnight fast. Eighteen men completed the study. Clinical evaluations and laboratory tests were done immediately before dosing and approximately 48 hr after dosing. ... Neotame was rapidly eliminated with a half-life ranging from 0.61 hr to 0.75 hr. The short half life was supported by the rapid disappearance of neotame from the urine (neotame was not detectable after 8 hr). ... The calculated half life of de-esterified neotame in plasma was approximately 2 hr.
WHO/FAO: Expert Committee on Food Additives. Summary of Toxicological Data of Certain Food Additives Series 52: Neotame (165450-17-9) (2004). Available from, as of July 18, 2011: https://www.inchem.org/pages/jecfa.html
The sweet taste receptor is a heterodimer of two G protein coupled receptors, T1R2 and T1R3. Previous experimental studies using sweet receptor chimeras and mutants show that there are at least three potential binding sites in this heterodimeric receptor. Receptor activity toward the artificial sweeteners aspartame and neotame depends on residues in the amino terminal domain of human T1R2. In contrast, receptor activity toward the sweetener cyclamate and the sweet taste inhibitor lactisole depends on residues within the transmembrane domain of human T1R3. Furthermore, receptor activity toward the sweet protein brazzein depends on the cysteine rich domain of human T1R3.
PMID:17168764 Cui M, et al; Curr Pharm Des 12 (35): 4591-4600 (2006)
The sweet protein brazzein [recombinant protein with sequence identical with the native protein lacking the N-terminal pyroglutamate (the numbering system used has Asp2 as the N-terminal residue)] activates the human sweet receptor, a heterodimeric G-protein-coupled receptor composed of subunits Taste type 1 Receptor 2 (T1R2) and Taste type 1 Receptor 3 (T1R3). In order to elucidate the key amino acid(s) responsible for this interaction, we mutated residues in brazzein and each of the two subunits of the receptor. The effects of brazzein mutations were assayed by a human taste panel and by an in vitro assay involving receptor subunits expressed recombinantly in human embryonic kidney cells; the effects of the receptor mutations were assayed by in vitro assay. We mutated surface residues of brazzein at three putative interaction sites: site 1 (Loop43), site 2 (N- and C-termini and adjacent Glu36, Loop33), and site 3 (Loop9-19). Basic residues in site 1 and acidic residues in site 2 were essential for positive responses from each assay. Mutation of Y39A (site 1) greatly reduced positive responses. A bulky side chain at position 54 (site 2), rather than a side chain with hydrogen-bonding potential, was required for positive responses, as was the presence of the native disulfide bond in Loop9-19 (site 3). Results from mutagenesis and chimeras of the receptor indicated that brazzein interacts with both T1R2 and T1R3 and that the Venus flytrap module of T1R2 is important for brazzein agonism. With one exception, all mutations of receptor residues at putative interaction sites predicted by wedge models failed to yield the expected decrease in brazzein response. The exception, hT1R2 (human T1R2 subunit of the sweet receptor):R217A/hT1R3 (human T1R3 subunit of the sweet receptor), which contained a substitution in lobe 2 at the interface between the two subunits, exhibited a small selective decrease in brazzein activity. However, because the mutation was found to increase the positive cooperativity of binding by multiple ligands proposed to bind both T1R subunits (brazzein, monellin, and sucralose) but not those that bind to a single subunit (neotame and cyclamate), we suggest that this site is involved in subunit-subunit interaction rather than in direct brazzein binding. Results from this study support a multi-point interaction between brazzein and the sweet receptor by some mechanism other than the proposed wedge models.
PMID:20302879 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879441 Assadi-Porter FM, et al; J Mol Biol 398 (4): 584-94 (2010)


